Guanidine hydrochloride didn’t just turn up in the science world overnight. Back in the late 19th and early 20th century, researchers started to pay attention to organic bases and their salts, searching for ways to untangle the building blocks of proteins and nucleic acids. Chemists quickly realized this compound offered a straight path into studying protein denaturation. It’s no surprise—guanidine itself caught the eye of Nobel laureates like Emil Fischer, who saw its potential in exploring peptidic structures. Over the decades, labs made it a staple for biochemical exploration, often as the reagent behind many protein folding studies. In my own lab days, guanidine hydrochloride always signaled a serious dive into protein sample prep and refolding trials—an essential, not a novelty.
Today’s market moves fast, but guanidine hydrochloride keeps holding its ground thanks to how it cracks open proteins for molecular biologists, geneticists, and even pharmaceutical manufacturers. Suppliers worldwide ship it as a white, crystalline powder, prized for its reliability and consistency. Laboratories everywhere rely on its effectiveness for cell lysis and RNA extraction, making it almost as common as sodium chloride on the shelf for certain specialists. Speaking with peers, I’ve found that once you figure out the quirks of working with it, you rarely look back. It often steps in where other denaturing agents fall short by giving a clean slate for enzymes and proteins.
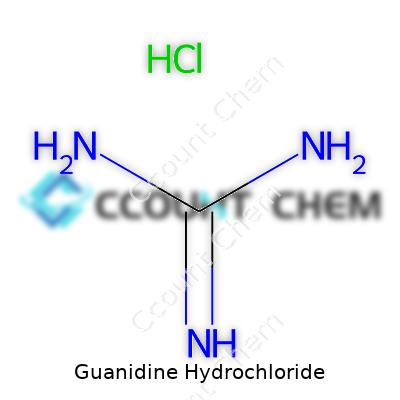
Guanidine hydrochloride stands up as a solid—literally and figuratively. It forms hygroscopic white crystals, melting around 182°C, and dissolves rapidly in water without fuss. The strong electrolytic nature and high basicity mean it loves to disrupt hydrogen bonds, making quick work of tightly packed proteins. Its simple formula, CH6N3Cl, belies the punch it packs on a molecular level. The compound brings a mildly bitter taste and slippery feeling when handled by hand—something anyone who has spilled a bit on the bench can confirm. Its pH-around-6 aqueous solution and reliable shelf stability make it a long-term ally for regular sample work.
Chemical suppliers don’t cut corners here. The purity level hovers at or above 99%, and researchers keep an eye on the source to avoid trace contaminants that can play havoc with delicate processes like PCR or cell culture. Each jar brings important labels: batch numbers, expiry dates, and hazard pictograms warning against contact and ingestion. Classification as an irritant reminds everyone to wear the right gloves and goggles without exception. My own best advice—always check the lot analysis before settling into a new batch, as batch-to-batch consistency often decides experiment outcomes, especially for clinical applications.
Most production labs use a reaction involving dicyandiamide and ammonium chloride under heat and pressure to yield the pure salt. Some processes go with cyanamide instead. Manufacturers control pH, temperature, and reaction time, later purifying with crystallization steps. Efficiency here isn’t just about volume—it can mean cleaner raw material, fewer residual byproducts, and a product that lives up to tight community standards. In small-scale academic labs, the practical route often comes down to buying rather than synthesizing due to waste management challenges and time constraints.
Chemists love how guanidine hydrochloride jumps right into reactions. It acts as a nucleophile, turns into guanidine derivatives through substitution, and can participate in methylation or other key functionalization processes. I’ve worked in protein chemistry setups where its denaturing action makes it a backbone of unfolding assays—after all, it leaves proteins dangling open for analysis or refolding studies. Guanidine hydrochloride partners up in organic syntheses to help generate isothiourea intermediates and fine-tune heterocyclic scaffolds, which pharmaceutical researchers value for drug design. It’s as versatile as any tool on the shelf, whether in an industrial reactor or an academic flask.
Everyone runs into a different name for this compound. My chemical safety sheets have listed everything from “guanidinium chloride” to “aminomethanamidine hydrochloride,” and product catalogs toss around abbreviations like GdmCl. Specialty suppliers sometimes dub it by brand or shorthand, especially for products destined for biochem or pharma work. It’s worth double-checking the structural diagram or CAS Registry Number—confusion over similar names can play tricks in a fast-paced lab.
Guanidine hydrochloride doesn’t play nice with bare skin or eyes. Direct spills on hands sting, and inhaling dust leaves you coughing for the rest of the day. Strong ventilation, gloves, and eye shields aren’t just suggestions—they’re the front-line defense against unnecessary risk. Regulatory authorities put a spotlight on secure storage: cool, dry places, tight containers, and good inventory records. Having handled it dozens of times, I always double-bag the stock and keep spill kits handy. Emergency protocols matter, especially with large-scale operators, where a small spill can escalate quickly.
Research scientists turn to guanidine hydrochloride for breaking down biological samples during DNA, RNA, and protein extraction. Labs count on it to strip away structure, denature nucleases, and prep pure, ready-to-study biomolecules within minutes. Clinical diagnostics teams reach for it during viral RNA extraction, crucial for PCR testing. Over the years, medical research has leaned on guanidine hydrochloride to help refold recombinant proteins for drug discovery, and the same holds in the food and environmental monitoring industries, where sample prep defines results. Some industrial cleaning processes leverage its ability to disrupt biofilms, and even hair care formulations occasionally dip into its stock for harsh straightening treatments—though not without concern over safety standards.
Scientific exploration keeps digging for new uses. Protein folding studies count on the precise denaturing action of guanidine hydrochloride, and single-molecule fluorescence techniques depend on exact concentrations to create the right experimental conditions. Genomics and proteomics workflows have evolved to demand higher purity and lower contaminant loads, spurring chemical vendors to offer ultra-pure and molecular biology-grade options. I’ve watched as new applications emerged in personalized medicine, especially in preparing samples for rapid diagnostic kits during public health emergencies, reminding me how essential adaptability is for research chemicals.
Animal studies and cell culture experiments underscore the risks of exposure—high concentrations put cells under stress or flat out kill them, making dosing accuracy essential. Chronic exposure has raised red flags over potential adverse effects on the nervous and cardiovascular systems, though acute toxicity tends to show its hand quickly, mostly as skin or mucous membrane irritation. Regulatory bodies in the US, EU, and Asia keep the compound under close evaluation in both environmental and occupational settings. I’ve always treated open containers with respect and double-checked ventilation after reading case studies where improper handling spelled trouble for both lab workers and nearby ecosystems.
Demand stands to stay strong as the push for rapid, reliable nucleic acid testing continues worldwide, especially for emerging pathogens and personalized health screening. Innovations in protein engineering and synthetic biology likely will lean further on compounds like guanidine hydrochloride to streamline workflows and boost purity. Chemists keep chasing greener synthesis methods and better waste recycling systems to lower the environmental footprint—something that matters more in the age of sustainable science. Automated platforms and high-throughput labs urge manufacturers to set even tighter impurity limits and provide robust documentation. My gut says tools like guanidine hydrochloride won’t fade soon; if anything, they’ll evolve to meet even higher standards for safety, reproducibility, and environmental protection.
Guanidine hydrochloride isn’t something most folks hear about outside a lab, though it’s been holding down key jobs in science and industry for decades. Describing it as a chemical seems incomplete—really, it's a heavy lifter in research, healthcare, and manufacturing, keeping a lot of modern life moving forward.
You’ll find guanidine hydrochloride in almost every DNA extraction kit. It works as a “chaotropic” agent. That means it breaks down proteins and helps open up cells, making it easier to pull DNA or RNA out of whatever sample you’ve got—blood, tissue, even soil. Without it, routine genetic testing becomes a much bigger mess, both in hospitals and crime labs. The stuff turns dense biological soup into a cleaner, easier solution for scientists to analyze. Ever since home DNA kits and rapid virus testing became affordable, demand has shot up. The pandemic spotlighted this role, especially when COVID-19 test labs ran through gallons of the chemical, freeing up viral material for faster, more accurate detection.
Every biology textbook points to proteins as the workhorses inside living things. Guanidine hydrochloride lets researchers unfold these complex molecules, not just for fun but so they can rebuild or understand what happens when proteins go wrong. This breakdown–refold cycle reveals how diseases like Alzheimer’s develop, since misfolded proteins play a big part. Having handled these procedures myself, I know nothing else works quite as reliably. Lab newcomers soon realize how much time they save with guanidine hydrochloride compared to older methods, which felt like doing a jigsaw puzzle in the dark.
Pharmaceutical manufacturers use guanidine hydrochloride to manage purity and performance, especially when working with complex proteins in drugs. Insulin and other biologic treatments come from living cells, which churn out proteins tangled up with stuff you don’t want in your medicine. This is where guanidine hydrochloride steps in to separate the good from the bad. Without this step, the risk of impurities grows, putting quality and safety on the line.
Take a look at certain disinfectants—guanidine hydrochloride sometimes shows up in compounds for cleaning equipment and surfaces in hospitals. Its protein-busting skill turns tough messes into broken-down bits the rest of the disinfectant can mop up. Hospitals want fast, reliable ways to keep bugs at bay, and this chemical fits that need. Some industrial cleaning processes also turn to it for similar reasons, giving medical and manufacturing teams safer workspaces.
No conversation about chemicals is complete without a nod to risk. Guanidine hydrochloride is strong stuff. Breathing it in or getting it on bare skin isn’t wise—lab veterans respect the gloves and goggles. Global guidelines require proper storage and disposal, since spills and careless use put both people and the environment in a bind. Too often, safety training gets skipped in the rush, but I’ve seen firsthand the value in regular refreshers and clear, ready-to-read safety sheets in every supply closet.
Some researchers push for greener alternatives. Recycling and smart sourcing help reduce the load on the environment. The push to use just enough—without wasting stock—can help labs cut their footprint, lower costs, and safeguard both workers and communities nearby.
Guanidine hydrochloride plays an outsized role in science and medicine, stepping in wherever rapid, reliable protein and genetic analysis sets the pace. Responsible handling and steady updates to training and sourcing practices are the way forward, keeping this chemical working hard for real people.Guanidine hydrochloride catches the eye of many lab technicians and scientists for good reason. It works wonders when breaking down proteins, separating nucleic acids, and preparing samples for genetic work. I’ve seen it earn its keep in molecular biology labs, but its usefulness often means many newcomers eventually ask the same question: how safe is it to work with?
Safety sheets warn about the dangers of guanidine hydrochloride for a reason. This white, crystalline chemical brings risks to both the skin and lungs. A careless spill on bare hands or a quick sniff near an open bottle may lead to burning, redness, or worse if the exposure runs long. Inhaling dust could trigger a sore throat, shortness of breath, and at higher doses, even more severe respiratory irritation. Every time I open a bottle of this compound, I remember the sore hands and mild cough from the one time I thought a quick bench-top transfer needed no gloves or mask.
It isn’t just about lab accidents: guanidine hydrochloride can reach the bloodstream if you forget its hazards or mix up lab routines. Swallowing—even tiny amounts—leads to nausea, stomach pain, and in rare cases, nervous system effects. Some studies connect long-term workplace exposure with effects on the thyroid in animals, which should be a loud warning sign for anyone who uses it often.
Data from the US Occupational Safety and Health Administration recommends limiting exposure to guanidine compounds. Fines have followed labs that failed to provide gloves, fume hoods, or adequate training. In my experience, tight quarters or under-ventilated spaces make dust and vapors much worse, so I always insist on a fume hood and goggles alongside gloves, even though the temptation to skip steps can sneak in during busy weeks.
New lab members sometimes treat common chemicals like they’re harmless, since the dangers don’t always hit right away. Training matters. I once watched someone try to save time by weighing guanidine hydrochloride in the open, only to spend the day with a red, itchy rash. The lesson stuck. Clear protocols and reminders help, but labs also need real-time supervision and reminders of past accidents. Sometimes, talking through the reality of exposure has a bigger impact than any printed instruction.
Simple fixes make a big difference. Always transfer and weigh guanidine hydrochloride under a fume hood. Use nitrile gloves, safety glasses, and lab coats every single time. Store it in tightly sealed containers and label the hazards clearly. If you spill any powder or solution, sweep up moist material with gloves and clean the area with plenty of water. Colleagues need to talk openly about close calls so each person learns the lesson before facing danger firsthand.
Guanidine hydrochloride earns respect, not fear. By facing the hazards and sticking to practical safety habits, this chemical can serve its scientific purpose without endangering health. I’ve learned to look past the routine—familiar chemicals bring just as much risk as the rare hazards. Pay close attention, use the right gear, and keep sharing what works and what has gone wrong in the lab. That shared lesson keeps everyone safe, no matter how many experiments line up for the day.
Guanidine hydrochloride pops up often in labs, especially for protein work and molecular biology. Anyone handling this chemical over time understands that messy storage leads to headaches—clumpy powders, mystery spills, or safety incidents. I’ve seen more than one researcher lose a batch to moisture or neglect.
Guanidine hydrochloride likes to absorb water from air, turning powder into sticky lumps. This hygroscopic tendency doesn’t just make it tough to measure—accuracy for protein denaturation really drops off if you ignore storage care. Even a cracked lid in a humid room ruins an expensive bottle in a week. A tight, tightly sealed container keeps the powder bone-dry. For extra protection, silica gel packets work wonders, drawing stray humidity away from the chemical itself.
Leaving guanidine hydrochloride on an open shelf near a window never seems like a big deal until sunlight breaks down the chemical or the heat causes slow degradation. Best results come from choosing a spot away from light and heat sources. Air conditioning in modern labs isn’t only about worker comfort; it actively slows down unwanted chemical changes. Good airflow also sweeps away any chance fumes collect unnoticed, which matters in tightly packed chemical storage rooms.
Every chemist who has ever reached into the wrong tray or worked near a cluttered fume hood has likely faced contamination risks. It doesn’t take long in the lab to learn that storing solids like guanidine hydrochloride away from solvents and acids prevents cross-reactivity. Separate shelves or storage cabinets cut down on mix-ups that lead to ruined experiments—or dangerous reactions.
Mislabeling seems like a rookie mistake, but even seasoned techs sometimes forget. A clear, legible label with the open date and hazard information heads off confusion—especially during busy times. Digital inventory systems also help manage expiration dates and order tracking, preventing those classic moments when the next experiment stops cold because the last of the chemical vanished weeks before.
Many commercial suppliers ship these bottles in double bags, highlighting just how easy it is for air to get inside. Following their lead, I always keep guanidine hydrochloride inside a sealed vessel, then place the vessel in a secondary closed container. This two-layer approach offers strong defense against leaks or accidents. Avoiding direct contact with water or solvents is essential—one spill across a storage shelf quickly contaminates everything close by. Personal safety means working with gloves and eye protection every single time, just in case fine powder escapes during retrieval.
Small steps add up. Rotating stock, limiting batch sizes in day-to-day work, and training every new lab member on correct procedures builds a solid habit that protects research integrity. Investing in purpose-built desiccators, properly maintained storage rooms, and good record-keeping pays for itself with healthier experiments and safer spaces. Lab safety officers or experienced peers can run regular checks, catching overlooked risks before they get expensive—or dangerous.
Paying close attention to proper storage prevents most of the classic failures with guanidine hydrochloride. In my experience, a bit of care turns an unpredictable reagent into a reliable tool. That careful mindset, more than any single storage trick, makes lasting success in any research setting possible.
Guanidine hydrochloride doesn't appear particularly sinister on a shelf. It serves as a useful chemical in biology labs for denaturing proteins and in RNA extraction. I’ve spent hours working with it during molecular biology protocols. One thing I always noticed—handling isn't difficult if you stay alert, but cleaning up presents a different challenge. Pouring leftover reagents or contaminated solutions down the sink feels like an easy habit, especially during busy evenings in graduate school. Still, even chemicals that seem mild can become an environmental headache when proper disposal gets ignored.
Anyone who’s checked a safety data sheet will see that guanidine hydrochloride brings its share of hazards. Being a strong denaturant, it carries toxicity for both people and aquatic organisms. That powder might not release clouds of fumes, but even small amounts slipping into water systems can hurt fish and disrupt wastewater treatment processes. The World Health Organization points out that improper lab chemical disposal ties directly to wider water contamination and ecosystem problems, not to mention chronic exposure risks for people who live around water sources.
Labs and companies working with guanidine hydrochloride fall under national, state, and municipal chemical disposal laws. In the US, the Environmental Protection Agency lists untreated guanidine waste as hazardous when it’s mixed with acids or oxidizers—which isn't rare in molecular biology work. Simply diluting the chemical or washing it away violates regulations and can lead to hefty fines for institutions. The burden typically falls on safety officers and environmental specialists, but individuals in the lab need to take ownership, too.
Growing up with a parent who worked in municipal water management, I frequently heard stories about laboratory chemicals disrupting downstream treatment. Guanidine-based waste sometimes deactivates certain strains of helpful bacteria used in water purification. What that means, practically, is more effort and money to keep tap water safe and clean.
Labs should always store guanidine hydrochloride waste in compatible, clearly labeled containers. The most responsible approach relies on collecting all liquid and solid waste, even small spills, and handing them over to certified hazardous waste services. These professionals render dangerous substances harmless using approved neutralization and incineration procedures. Keeping chemicals out of general trash prevents accidental exposure during transportation and disposal.
Some universities now track every step of chemical disposal using electronic logs, making individual researchers accountable. Waste auditing and regular training sessions help keep safety fresh in everyone’s mind. There’s also a push for greener protocols in life sciences. Labs increasingly lean toward enzymatic or in vitro systems that require fewer harsh chemicals, which naturally reduces dangerous waste.
No magic bullet wipes out the risks posed by guanidine hydrochloride, but small habits define the safety culture. People who treat chemical waste with care do more than tick a compliance box—they help protect their coworkers, neighbors, and the rivers where kids fish in summer. I always remind new lab members: check the protocol, follow the disposal guides, and never guess what goes down the drain.
Improving chemical safety starts with practical knowledge and shared commitment. By respecting guanidine hydrochloride’s risks, both labs and communities stand to benefit. Let’s not ignore the lessons written in our water and soil; simple steps today save headaches, costs, and lives tomorrow.
Anyone handling proteins or working on RNA extraction probably knows guanidine hydrochloride. This powder plays an important role in keeping proteins unfolded or breaking open stubborn cell walls. Purity isn’t a feel-good certificate on a bottle; it’s a real concern for scientists who care about their data, their safety, and the results they hang their names on.
I've spent late evenings watching an experiment go sideways, later realizing the only variable was a reagent’s impurity. Guanidine hydrochloride isn’t different. Labs depend on it in pure form because any impurity could mess with protein structure, create phantom bands on gels, or make an RNA prep worth tossing out. Most suppliers push “Molecular Biology Grade” or “Ultra Pure” labels — but those lines don’t mean much without real numbers behind them.
For guanidine hydrochloride, purity climbs above 99%, often touching 99.5% for legitimate research use. Reputable sources share detailed specs — total organic content must stay low, and some even state sulfate presence below 0.01%. Iron and heavy metals need tight control, measured in tiny slivers like 0.0005%. Chloride content stays above 19% to match the molecule. Even the moisture must fall under 0.5%, since excess water sneaks in problems for protein work.
Those numbers don’t just come from clipboard checklists. A contaminant, like heavy metals, can kill sensitive enzymes or alter protein folding. Organic contaminants drift in from manufacturing shortcuts or lazy storage and end up throwing off analytics. In teaching labs, a bottle of impure guanidine hydrochloride just means students waste time guessing wrong about what failed. In clinical and pharma settings, a loose batch can land a project’s credibility in the trash.
Some years ago, I worked with a batch that was nearly 98% pure — seemed okay until our mass spec lit up with unexplained peaks. Cost-saving on chemicals turned the week into a paperwork nightmare. It sounds boring to push for better specs, but this is the backbone of repeatable research. Public trust relies on clear, reproducible findings. Regulators want proven control over every input, especially in anything related to medicine.
Reliable suppliers offer a Certificate of Analysis with every batch, tracked by lot number, not just a generic data sheet. I’ve learned to ask for HPLC or titration data — one piece of paper can mean the difference between publishable results and hours lost to troubleshooting. With the rise of global sourcing, some labs slip in cheap reagents and hope nobody notices. That short-term win usually ends up more expensive, thanks to failed experiments and lost time.
Labs can call out suppliers on transparency. Ask for the actual analysis, not just the word “pure” in bold font. Peers should swap stories about what works and who delivers consistent quality. Universities and companies can set minimum acceptance criteria for this and other core reagents, creating a floor that everyone benefits from.
The bottom line? There’s no shortcut around purity specs in guanidine hydrochloride. Reliable science, sensible budgets, and even health outcomes trace back to what’s actually in that white bottle. The best researchers I know refuse to cut corners on sourcing and always trace problems to the tiniest sources. It’s not just about numbers on a page — it’s about everything that comes next.
| Names | |
| Preferred IUPAC name | guanidinium chloride |
| Other names |
Guanidinium chloride Aminomethanamidine hydrochloride Guanidinium chloride Guanidinium monochloride Guanidini hydrochloridum |
| Pronunciation | /ɡwəˈnɪdiːn haɪˈdrɒklaɪd/ |
| Identifiers | |
| CAS Number | 50-01-1 |
| Beilstein Reference | **83652** |
| ChEBI | CHEBI:35918 |
| ChEMBL | CHEMBL1422 |
| ChemSpider | 14106 |
| DrugBank | DB11345 |
| ECHA InfoCard | 100.032.028 |
| EC Number | 207-312-8 |
| Gmelin Reference | 47344 |
| KEGG | C01881 |
| MeSH | D006147 |
| PubChem CID | 3520 |
| RTECS number | MF4300000 |
| UNII | FD09Q851ZV |
| UN number | UN1759 |
| CompTox Dashboard (EPA) | DTXSID8021152 |
| Properties | |
| Chemical formula | CH6ClN3 |
| Molar mass | 95.53 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.2 g/cm³ |
| Solubility in water | 224 g/100 mL (20 °C) |
| log P | -1.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 11.5 |
| Magnetic susceptibility (χ) | -6.2e-6 |
| Refractive index (nD) | 1.382 |
| Viscosity | 1.74 cP (20 °C, 6M solution) |
| Dipole moment | 6.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –132.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -132.1 kJ/mol |
| Pharmacology | |
| ATC code | D06BA08 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | 80 °C |
| Autoignition temperature | 400°C (752°F) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat 475 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 626 mg/kg |
| NIOSH | RX8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 500 g |
| IDLH (Immediate danger) | 500 mg/m³ |
| Related compounds | |
| Related compounds |
Amidines Formamidine Methanediimine Methylguanidine Thiourea |