Chemistry has always leaned on the fine details, and the story of ethanesulfonic acid isopropyl ester shows how small tweaks in molecules shift whole fields. Decades ago, as labs dug into sulfonic acid derivatives, they found themselves drawn to the possibilities of altering the sulfonate group. Adding an isopropyl ester brought cleaner reaction paths and steadier yields, sparking an uptick in research. In university libraries, I used to pore over old journals tracing how the postwar chemical boom drove specialty reagents into focus—this one quietly worked its way from academic shelves into pilot-scale manufacturing. Each step depended on pushing the frontier a bit further, leaning on new purification tricks and analytic techniques. Sitting in a lab, watching reactions turn and waiting for GC-MS data, you start to appreciate how simple molecules like this one become workhorses for dozens of pathways in synthesis.
Today, ethanesulfonic acid isopropyl ester lines up as a clear, slightly oily liquid that bridges the gap between laboratory specialty and industrial practicality. Chemists reach for it in selective alkylation or as an intermediate. Product specs matter a lot here—the industry learned fast that margins for impurities are thin. Producers keep water content below 0.5%, and even tiny traces of byproducts can break a synthesis downstream. In catalogues and on safety sheets, its identities shift—sometimes it goes by isopropyl ethanesulfonate or 2-propanol ethanesulfonate, reflecting the stubborn creativity chemists bring to naming conventions.
No mystery hides in the appearance: colorless, faint odor, density close to 1.1 g/cm3. Its boiling range stretches upwards of 200°C, and it's soluble enough in common organic solvents to keep it mobile during reactions. Many folks outside the field don’t pause over a molecule’s polarity, but any chemist running a separation knows details like this can save hours in the lab. I’ve seen mistakes pile up just because someone skipped a look at miscibility or underestimated volatility under reduced pressure. Its stability mostly holds under typical storage, provided you dodge moisture and strong bases; hydrolysis creeps in otherwise, breaking apart the ester and spoiling the batch.
Manufacturers lean hard on clear labeling: CAS numbers, batch codes, purity, and storage instructions take up prized space on every drum and bottle. Country regulations lay out their own hurdles—REACH makes European labels read like whole encyclopedias, while US shipments include HMIS and GHS symbols. Each label does more than fill compliance checkboxes. It’s the lifeline for safety in shipping and storage and tracks accountability when things go sideways. Working in QA, I learned that a single typo can trigger a scramble if a safety board or customs spot an inconsistency. The right label, in the right place, keeps both people and shipments moving.
Manufacture tends to follow routes familiar to anyone who’s tried big-scale esterifications. Ethanesulfonic acid reacts with isopropyl alcohol—acid catalysts push the process, pulling water out to drive completion. Commercial production swaps in azeotropic distillation or molecular sieves to beat down water levels, protecting yield and purity. Some processes tweak catalysts or use sulfonyl chlorides, trading speed for fewer side reactions. Every plant and pilot lab finds their own balance, usually based on raw material costs or waste treatment requirements. In small-scale academic setups, I’ve run these methods with shaking hands, watching tempers flare when water control slips—scaling up turns those personal annoyances into expensive process bottlenecks.
Ethanesulfonic acid isopropyl ester walks into all sorts of transformations. It acts as an electrophile in SN2 reactions, making it useful for introducing ethylsulfonate groups onto nucleophilic centers in pharmaceutical intermediates or specialty polymers. I’ve watched colleagues seize on its reactivity to crack some stubborn synthetic routes; the molecule slips into pathways for constructing protective groups, too. Handling brings its own complexity. Strong bases break it apart—so you plan your workups with care, controlling pH and monitoring for traces of hydrolysis. Labs tinkering with its structure often build bulkier or more reactive substitutes, tweaking properties like leaving group ability or solubility for new process windows.
Chemists love alternate names, sometimes to the point of confusion. Ethanesulfonic acid isopropyl ester migrates across bottles as isopropyl ethanesulfonate, 2-propyl ethylsulphonate, or even sulfoethanolic acid isopropyl ester. Catalogues sometimes list regional trade names, especially from Eastern Europe or East Asia, which helps or confuses purchasing in equal measure. Lab teams double-check synonyms not just for ordering, but to avoid incompatible substitutions—mistakes here waste both time and budget.
Every batch reaching the lab or plant triggers a quick review of the Material Safety Data Sheet. Its moderate toxicity and potential for irritation demand respect. Spills smell faintly, but skin and eye exposure mean running straight for the eye wash or safety shower. Like many organosulfonates, this compound doesn’t explode but doesn’t play nice with oxidizers or open flames. Air circulation, gloves, and goggles stay mandatory even during routine handling. OSHA guidelines and country-specific worker safety laws cover the basics, but smart teams bring in local practices, like secondary containment and double-bagging waste. I’ve seen near-misses where someone skipped decontamination; those stories echo through teams and drive home how unforgiving chemical operations can turn if safety slides.
You’ll find this molecule tucked into labs working on pharmaceuticals, dye intermediates, and specialty monomers. Pharma process engineers covet it for clean alkylation steps while materials scientists value its role introducing sulfonate groups—those improve conductivity or modulate solubility in polymers and resins. In one project, its use streamlined a late-stage functionalization, shrinking timelines and cutting purification cycles in half. Environmental chemists toy with its breakdown and adsorption, eyeing impacts in wastewater streams. Academic groups run kinetic studies or mechanistic probes, tracing how each molecular nudge changes the reaction outcome. No one role defines it, but dependence on consistent purity and reactivity shows up in every success or bottleneck story.
The front edge of research keeps looking for new tricks with this compound. Engineers test greener synthesis methods—biocatalysts, solvent swaps, and closed-loop recycling stand out as current targets. Cross-disciplinary projects examine novel polymer backbones, letting this ester seed reactive handles. Cheminformatics teams model its pathways, feeding the results back to scale-up teams gunning for fewer byproducts or single-pot syntheses. At R&D roundtables, I’ve watched innovation surface from both theory and hands-on failures—new impurities, odd reactivity, or unexpected stability each send teams back to the drawing board, forging better controls or alternate routes. Funding follows applications into pharma and electronics, securing a future for this molecule beyond its decades-old reaction schemes.
Toxicology studies pin down the real-world hazards far better than sweeping theory ever could. Inhalation, skin absorption, and accidental ingestion all show moderate risks—lab work shows temporary irritation, but chronic data remains limited. Animal studies note hepatic and renal strain at higher exposures, pushing regulatory bodies to recommend strict exposure limits and personal monitoring for workers. Environmental fate studies keep stress-testing its breakdown in water and soil, looking for persistent metabolites or unexpected byproducts. I’ve seen risk assessments shape entire hazard communication strategies; more than a few product launches paused until safety and environmental data told a clearer story. Data gaps still matter—so ongoing studies grab funding and attention from public health and chemical safety groups alike.
Market observers watch evolving demand from pharma, coatings, and advanced materials as regulatory and customer standards climb ever higher. Sustainability targets push the industry to cut down on waste and boost green chemistry credentials, so development teams prioritize new catalytic cycles and eco-friendly solvents. Smart process integration aims to notch up yields and shrink footprints, letting this humble ester sit at the intersection of quality, safety, and innovation. I expect continued pressure for transparency on sourcing and toxicology, with digital tracking of batches and real-time safety data updating everything from registries to procurement. New properties—higher selectivity, temperature range, or tailored reactivity—could open further market slices in electronics, battery chemistries, or next-gen adhesives. Companies and universities with the grit to keep asking hard questions will find more to do with ethanesulfonic acid isopropyl ester for years ahead.
People don’t bump into ethanesulfonic acid isopropyl ester in daily life, and it rarely shows up in science classes at school. Step into a research lab, though, and you’ll notice bottles labeled with tongue-twisters like this one. Beneath the complex name is a clear purpose: it plays a key part in making chemical reactions work smoothly, and sometimes only this specific chemical gets the job done.
In labs where scientists make new molecules for medicine, ethanesulfonic acid isopropyl ester acts as a strong agent for transferring groups called sulfonates. What that means in practice: the compound helps put together pieces of molecules in just the right way, especially when building medicines, crop protection products, or specialty materials. Instead of forcing a reaction to take the hard road, researchers use this ester to take a shortcut—saving both resources and time.
Pharmaceutical companies turn to reagents like this ester because many medicines need special features tucked into their structure for stability or targeted action in the body. Chemists discovered that adding sulfonate groups can make a drug dissolve better or help it move through the body more predictably. Ethanesulfonic acid isopropyl ester makes it simpler to add those groups, especially to complex molecules that don’t give up their secrets easily. It’s not in the pill bottle you get from the pharmacy, but it helps make sure what’s inside actually works.
Many scientific papers come together only after mountains of trial and error. Having a clever reagent can make or break a discovery, especially in industries racing for patents. Schools often brush past how difficult it is to build specific parts onto molecules without unwanted leftovers. This is where the ester shines. By giving chemists a reliable way to avoid side reactions or unwanted by-products, it opens doors to new compounds that might lead straight to the next big medical breakthrough.
Working with ethanesulfonic acid isopropyl ester is not a casual affair. It packs strength, so safety matters—a lesson every young chemist learns fast. Protective gear, careful storage, and close attention to procedures keep people safe and prevent costly mistakes. Mishandling chemicals like this risks health or ruins expensive materials, so the rules stand for good reason.
Chemical research leaves a footprint. Responsible professionals think about where reagents end up after reactions finish. Proper disposal and containment of ethanesulfonic acid isopropyl ester safeguards water, air, and soil from contamination. Regulations require detailed tracking, and ethical labs go further. Finding ways to use less, recycle, or swap out hazardous chemicals pushes the field toward better, cleaner science.
Most people never see or hear about reagents like ethanesulfonic acid isopropyl ester, but their impact runs deep. The products on pharmacy shelves, the treatments in hospitals, and the materials powering everyday devices started in a lab with reagents making hard reactions easier. The bottom line is that progress in science often relies on invisible aids like this one.
Ethanesulfonic Acid Isopropyl Ester can create real hazards if someone handles it carelessly. Anyone who's spent time with this chemical knows its risks go past just a splash on a lab coat. It releases toxic fumes, burns skin, and eats through clothing if you don’t have the right gear. Accidents don’t just hurt people—they disrupt research, rack up costs, and bring regulators down with inspections no one wants.
Gloves made of nitrile or neoprene offer a reliable line of defense. Latex won’t cut it with strong acids. Splash goggles keep eyes safe; regular glasses leave the sides exposed, so liquid can still reach your skin. A face shield helps when pouring or transferring in larger quantities. A flame-resistant lab coat does more than just meet the dress code; it protects from splashes and chemical burns. It pays to wear closed shoes, too. After witnessing an accident where a colleague suffered deep burns through sneakers, I never forget steel-toed shoes or rubber boots.
A fume hood keeps vapors from escaping into general lab space. Once, I walked into a room where someone opened this acid on a benchtop instead of in a hood, and in under a minute, breathing got uncomfortable, eyes stung, and the whole lab had to evacuate. So, make sure the ventilation works. Chemical-resistant trays or secondary containers trap spills. I’ve seen a plastic tray save a granite countertop from a chemical etching disaster.
The Safety Data Sheet (SDS) for Ethanesulfonic Acid Isopropyl Ester breaks down the risks, like corrosivity and flammability. One overlooked hazard—some esters give off toxic or flammable fumes if heated or mixed with incompatible chemicals. Acids and bases stored too close create explosions. Many labs hang incompatibility charts on the wall, a smart measure. Lab workers learn early: don’t trust your memory—check the chart each time.
Once, a bottle broke during a rush. The difference between panic and control came down to regular practice. Neutralize spills with a proper acid spill kit—not just baking soda from the break room. Ventilate if fumes seem strong. Remove contaminated clothing. Dispose of waste by chemical guidelines, not just down the sink. At my last workplace, we checked emergency showers monthly because one failed right when we needed it most.
Keep this ester in tightly closed containers, away from moisture and strong bases. Store acids on low shelves, never above eye level. A neighbor lab once stacked acids overhead, and a dropped bottle caused cascading breakage—a mess no one wants. Use labels with clear print, including the hazard class, to prevent confusion.
Lab safety isn’t just paperwork. Training sessions and surprise drills build habits that stick. Encourage questions during sessions, since people remember hands-on practice much more than reading a policy manual. Stress and fatigue make mistakes more likely, so supervisors set up checklists and buddy systems for tasks involving dangerous acids.
No one wants to sound alarmist, but speaking up about unsafe storage or missing spill kits stops accidents before they spread. Most injuries aren’t caused by lack of knowledge, but by letting little habit slips pile up. Safe handling of Ethanesulfonic Acid Isopropyl Ester comes from respect—learned the hard way by those who have seen just how dangerous it gets without it.
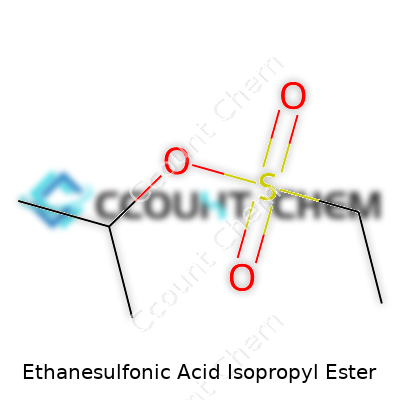
Many folks in chemistry labs and research sites run across unfamiliar names like Ethanesulfonic Acid Isopropyl Ester. Breaking down the formula helps demystify things. This compound is built from a sulfonic acid core attached to ethane, then capped off with an isopropyl group. Structurally, its formula comes out as C5H12O3S. The puzzle fits like this: the ethane portion (C2H5), the sulfonic acid (SO3H) becomes SO3, and then gets an isopropyl group (C3H7) as the ester. Scientists use a formula like this to calculate, predict, and analyze. The molecular weight, if you add up all the atomic masses, settles at about 152.21 g/mol.
A lot of folks outside the lab don’t realize how a small detail, like molecular weight, can flip the outcome of an entire project. Handling a compound such as Ethanesulfonic Acid Isopropyl Ester, knowing the formula and weight isn’t trivia—it’s the gateway to safe handling and correct dosing. There’s not much room for guessing, especially when pharmaceutical researchers design salts to ensure that drugs dissolve at the right rate in water. Get the math wrong, you court disaster for patient safety and the company’s reputation.
My early days in chemistry reminded me every day that shortcuts cost more than they save. Working once with similar sulfonic esters, a trusted team member relied on an outdated reference table—nothing catastrophic, but the results in our synthesis drifted beyond spec. That delayed the project and left everyone double-checking what should have been basic data. After that, we all developed a respect for checking and cross-checking formulae and weights before moving down the bench or onto the scale.
Focusing on accuracy might sound fussy, but for industries like pharmaceuticals and materials science, it steers projects clear of regulatory nightmares. Researchers can’t claim high-quality products without pinpointing the chemicals involved. Mislabel a bottle or botch measurement, it clouds the downstream analysis, slows regulatory approvals, or even risks health. Companies invest a fortune to steer clear of recalls or regulatory fines just because somebody skipped the basics.
The thirst for quality doesn’t only spring from compliance. High-purity reagents like Ethanesulfonic Acid Isopropyl Ester turn up in complex syntheses and custom chemical processes. Every mol of the stuff needs to match precisely what's in the handbook, not just what looks right on paper or appears clean in the bottle. This attitude didn’t just improve my own record-keeping; it made partners and auditors breathe easier during reviews and audits.
The best way forward for anyone working with fine chemicals: take nothing for granted. Always confirm chemical formulae—such as C5H12O3S for Ethanesulfonic Acid Isopropyl Ester—before use. Double-check molecular weights (152.21 g/mol in this case) instead of trusting memory. Encourage open communication and habits that put safety and precision before convenience. Whether you’re on the bench or behind a desk, reliable data builds trust, delivers better science, and keeps business running smoother than any shortcut ever could.
Anyone who has worked in a lab knows that chemicals can turn into hazards if storage guidelines aren’t followed. Ethanesulfonic Acid Isopropyl Ester raises some specific storage concerns due to its reactivity and possible health impacts. Getting this right avoids both material loss and unnecessary risk.
Glass containers, with a good seal, give more reliability than plastics for this compound since reaction with some plastics can’t be ruled out. Sturdy glass bottles, with screw caps lined with PTFE, stop air and moisture. The container should sit upright.
On the shelf, avoid storage above head height. If the bottom shelf works, pick it. In my old lab, someone once bumped the shelving; saved a major clean-up only because acids sat close to the floor.
Heat speeds up decomposition in sensitive chemicals. A spot with consistently cool temperatures, ideally below 25°C (77°F), avoids breakdown. Even small chemical fridges work in resource-limited settings. Keep the compound out of sunlight as UV can act as a trigger for changes in molecular structure—opaque or amber bottles cut down light exposure.
Do not let it freeze unless usage data says otherwise; freezing can crack glassware and humidity from condensation only adds issues.
Moisture in the air reacts with sulfonic acids and related esters. That means a dry spot is not just ideal; it helps preserve quality. In one instance, high humidity ruined a batch intended for an important analysis, which forced a re-order and lost an entire week. Desiccators help, especially where coastal humidity gets into everything. Keep the lid screwed on as soon as you’re done pouring.
Low oxygen environments cut risks in scale-up settings, but in most labs, airtight glassware with no leaks goes a long way. Leaving bottles open, even for a minute, gives moisture and air just the window they need.
Sharp labeling prevents mistakes. Include name, concentration, storage date, and your initials for full traceability. Even experienced staff can confuse similar bottles, particularly during busy shifts. Never store next to strong oxidizers or alkali substances—mixing accidents create major hazards. Flammable or corrosive cabinets, depending on local regulations, add another layer of safety.
Spill kits nearby, plus clear instructions, cut response times if things go wrong. Wear gloves, goggles, and long sleeves during handling, since even small splashes can burn skin or eyes.
Safe chemical storage comes down to habits and vigilance. Labs that keep clear protocols, train new team members, and routinely check inventory avoid most pitfalls. Updates on best practices—drawn from real accidents and successes—help everyone. A culture of safety keeps both people and data secure.
A chemical like ethanesulfonic acid isopropyl ester might seem niche, but its backstory repeats a pattern anyone in research or manufacturing recognizes: purity runs the show. In the lab, nobody wants to discover their hard-earned data got bumped around by an impurity. Over time, I’ve watched teams pour hours into troubleshooting experiments, only to learn that a reagent slipped in with the wrong grade. One small detail, big impact.
Most suppliers don’t stick to a single grade. Lab-grade, technical, and high-purity options reflect different needs. The grade stamped on that bottle signals its trustworthiness for the job. Figuring out which one works always starts with what you’re building—drug synthesis, specialty coatings, or something entirely different.
For pharmaceuticals and biotech, that stamp usually means 98% purity or higher. Any less, and you’re gambling with safety, shelf life, and regulatory headaches. For less sensitive work—maybe in a chemical plant, or with some industrial reaction—the technical grade can make sense. This stuff might land between 90 to 95%. It’s easier on the wallet but might bring a few extra compounds along for the ride.
Regulatory bodies care about what goes into medicines and food. In my own work, I’ve seen the paperwork get intense, especially when dealing with substances for human or animal consumption. Certificates of Analysis, batch records, even traceability down to the lot—no shortcuts. Not every supplier can play in that sandbox.
Choosing the right grade isn’t just a scientist’s problem; it’s a legal and ethical one too. In regulated industries, impurities don’t just risk poor results; they could hurt someone. Companies look for detailed documentation and often audit suppliers to verify claims. For folks new to buying chemicals, that’s the moment to slow down and ask for proof before the purchase.
Global events—weather, pandemics, trade shifts—sometimes send chemical supplies off track. I’ve watched pricing and availability swing wildly in those moments. High-purity batches become scarce. Sometimes, the only stock on offer is technical grade, brought in from halfway around the world. Suddenly, purity isn’t just a technical question, it becomes a logistical puzzle. A product that seemed consistent for years can shift in quality when demand spikes or regulations tighten.
Improved transparency across the supply chain would take out a lot of guesswork. Real access to batch data—ideally shared in digital form—would let buyers know what they’re getting into, right at the quote stage. Routine third-party testing can help, too. That means suppliers need to work with accredited labs, not just their own in-house checks.
For small labs, pooling purchases to secure higher-quality chemicals at better rates often makes sense. It pays to keep relationships strong with reliable distributors, so surprises stay limited. If a project can’t afford to risk a batch, always pay for a higher grade and double-check the paperwork. Missteps in quality control rarely end well, and the fallout for cutting that corner runs high.
| Names | |
| Preferred IUPAC name | Isopropoxyethanesulfonic acid |
| Other names |
Isopropyl ethanesulfonate 2-Propanol ethanesulfonate IPES Isopropyl ethane sulfonate Ethanesulfonic acid, 1-methylethyl ester |
| Pronunciation | /ˌɛθeɪn.sʌlˈfɒn.ɪk ˈæsɪd ˌaɪ.səʊˈprɒp.ɪl ˈɛstər/ |
| Identifiers | |
| CAS Number | 4337-75-1 |
| 3D model (JSmol) | Ethanesulfonic Acid Isopropyl Ester (Isopropyl ethanesulfonate) – JSmol 3D model string: ``` CCOS(=O)(=O)C ``` This is the **SMILES** string for the molecule, which can be used in JSmol and other 3D visualization tools. |
| Beilstein Reference | 1699071 |
| ChEBI | CHEBI:51635 |
| ChEMBL | CHEMBL523876 |
| ChemSpider | 197471 |
| DrugBank | DB14096 |
| ECHA InfoCard | 100.026.608 |
| EC Number | 240-321-4 |
| Gmelin Reference | 67782 |
| KEGG | C19229 |
| MeSH | D017220 |
| PubChem CID | 136895046 |
| RTECS number | KY9250000 |
| UNII | 23G8Y2N9HQ |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID8051071 |
| Properties | |
| Chemical formula | C5H12O3S |
| Molar mass | 168.22 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Odorless |
| Density | 1.025 g/mL at 25 °C |
| Solubility in water | soluble |
| log P | 0.6 |
| Vapor pressure | 0.1 mmHg (20 °C) |
| Acidity (pKa) | 1.86 |
| Basicity (pKb) | 13.1 |
| Magnetic susceptibility (χ) | -7.63×10^-6 cm^3/mol |
| Refractive index (nD) | 1.4080 |
| Viscosity | 5 mPa·s (25 °C) |
| Dipole moment | 3.32 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -600.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -929.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | C01DX16 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H302 + H314 |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-1 |
| Flash point | 68°C |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 570 mg/kg |
| NIOSH | GV9450000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Ethanesulfonic Acid Isopropyl Ester is not specifically established by OSHA. |
| Related compounds | |
| Related compounds |
Methanesulfonic acid isopropyl ester Ethanesulfonic acid methyl ester Ethanesulfonic acid ethyl ester Propane-1-sulfonic acid isopropyl ester Ethanesulfonic acid tert-butyl ester Ethanesulfonyl chloride Isopropyl sulfonate |