Chemistry never stands still. Scientists began exploring sulfonium salts well before most of us learned our periodic table, driven by curiosity about their impact on reactivity. By the late 1900s, researchers dug into the realm of perfluoroalkyl sulfonates, and if you look at patent activity through the 2000s, interest in tailor-made ionic species saw new heights. Labs worldwide saw new ways to manipulate cationic and anionic components, betting on these molecules to solve real-world technical hurdles. Dimethyl(phenyl)sulfanium perfluorobutanesulfonate arrived as part of this trend, leveraging known effects of fluorinated tails on conductivity and stability. In those days, the code of "greener" and more robust compounds gained traction, despite the competition from older, less modified sulfonium salts.
What sets this compound apart isn’t just the sulfonium cation or the perfluorinated sulfonate anion by itself—it’s the combination. On the bench, you see a pale, crystalline or powdery material showing impressive solubility in polar solvents. The package often comes with moisture-tight seals: the material can be sensitive. A diversity of suppliers now ship this salt in research and commercial grades, some working closely with electronics and polymer industries. You can spot both small glass bottle packaging for small-scale R&D and kilogram drums for process development. The main draw remains the matched blend of electrochemical function and chemical ruggedness, a challenge for older classes of ionic compounds.
The chemical formula alone—C10H15FSO3—begs for a closer look. In the hands, the powder feels slightly slick, an echo of its fluorinated tail. This salt doesn’t just dissolve in water; it answers best to solvents like acetonitrile or dimethyl sulfoxide. Melting point tends to hover around 140-160 °C, showing stability under moderate thermal loads. Thanks to the perfluorobutanesulfonate anion, chemical inertness goes beyond what you get with plain chlorides or tosylates. The surface tension and wettability differ from non-fluorinated salts, and conductivity stands out, especially in lithium-free electrolytes. Over time, storage under dry inert gas gives the best shelf life, and long exposure to light or moisture leads to slow, measurable hydrolysis.
Quality is front and center in the specifications sheet. Leading suppliers often specify a purity greater than 98%, measured by NMR and HPLC. Water content rarely exceeds 0.1%—for sensitive applications like battery research, even less gets demanded. The labeling covers not only batch number, net weight, and lot details, but signals about transport and storage, including "Keep Dry, Store Below 25 °C." Regulations call for compliance with local and international chemical safety codes. Drivers in the supply chain log lot tracking for traceability. This kind of attention isn't just bureaucracy: end users want to trace back origins if equipment or experiments run into trouble.
Lab workers learn quickly that mixing methyl iodide with diphenyl sulfide, under basic conditions and careful temperature control, produces the dimethyl(phenyl)sulfanium cation. To attach the perfluorobutanesulfonate, anion exchange comes into play. Typically, this involves refluxing the intermediate salt with an excess of sodium perfluorobutanesulfonate in a polar organic solvent. Filtration and crystallization follow, and extensive washing with cold acetonitrile purifies the product. Handling methylating agents and sulfonate sources takes mighty care—proper ventilation and skin protection are non-negotiable. Scaling up this technique means constant vigilance: runaway exotherms risk both worker and facility safety. Constant documentation of yields, purity, and side-products guides continuous improvement in fine-tuning the process.
My own run-ins with this salt highlight its stability, but chemists do find ways to push its limits. Nucleophilic attack on the sulfonium core remains possible with powerful nucleophiles: thiolates and cyanides warrant care. Modification often means swapping the perfluorobutanesulfonate for longer or shorter perfluoroalkylsulfonates, tuning solubility and electrochemical behavior. Advanced synthetic teams sometimes graft bulky side chains onto the phenyl ring, giving new handles for cross-coupling or metathesis. In all this, the challenge stays the same—balancing reactivity with resistance to decomposition, especially for high-value electronic uses. Tests for degradation typically run under stress: heating, irradiation, strong acid or base. The results look promising, but quality control keeps a close eye on any trace ring-opening byproducts or fluorinated impurities.
Depending on where you look, this salt answers to a handful of names. Many catalog lists use "Dimethyl(phenyl)sulfonium perfluorobutanesulfonate" or "DMPS PFBS." In some literature—including patents and regulatory filings—phrases like "Me2(Ph)S PFBS" show up. A number sticks too: CAS 749906-77-4 gets cited regularly in regulatory texts. Industry newsletters and supplier brochures may abbreviate even further: "sulfonium PFBS" under a proprietary brand, linked to vendor-specific grades or application areas.
Anyone who has worked with sulfonium salts knows the safety dialogs aren’t for show. The acute toxicity looks lower than older alkylating agents, but gloves and goggles form the uniform. Standard operating procedures call for working in well-ventilated hoods. Emergency eyewash and showers must sit close by. If the powder spills, dry methods for clean-up beat water—hydrolysis creates byproducts that complicate disposal. Some jurisdictions want full compliance with REACH or TSCA rules, especially for compounds featuring perfluorinated groups. Routine workplace monitoring limits exposure to fine powders, and personnel log all incidents for internal review. Waste handling takes real attention: disposal goes by incinerator, not landfill, under the guidance of dedicated environmental safety officers. Regular refresher training keeps frontline workers updated when new toxicity findings hit the literature or guidance shifts in response to regulatory review.
Electrochemistry labs have given this salt space on their shelves. Its unique ionic structure improves ionic conductivity in non-aqueous media, a quality battery developers appreciate. Polymeric material specialists find use for it as an initiator or dopant, shaping conductivity in flexible circuits and sensors. Analytical chemists reach for dimethyl(phenyl)sulfanium perfluorobutanesulfonate when they want a noncoordinating counterion, something that won’t cloud up the signal in mass spectrometry or chromatography. In water treatment R&D, its extreme inertness draws interest for separating other fluoro-organic contaminants. I have seen collaborations spring up between materials scientists exploring next-generation ionomers and pharma groups looking for custom ionic triggers. The product fits into a niche technologies space, far from the likes of table salt but close to the cutting edge of energy storage and separations research.
Academic journals and patent filings show a steady rise in uses for this sulfonium salt. Recent studies document its behavior in artificial ion channel models and its role in membrane transport systems. Papers out of Asian and European universities dig into blends of PFBS-based salts in the hunt for safer electrolytes for lithium- and sodium-ion batteries. Process chemists explore milder synthesis conditions to cut overall costs, cut out hazardous reagents, and open doors for scale-up. Collaborations with industry keep pushing for new formulations: forklift battery manufacturers, electronic ink developers, and even groups testing water desalination all report pilot tests using this ionic compound as either a core component or performance booster. The research rarely travels in straight lines—after initial success with conductivity, teams often backtrack to adjust purity standards or switch supporting solvents as new data comes in.
Toxicologists and environmental scientists hold a microscope to perfluoroalkyl substances. Dimethyl(phenyl)sulfanium perfluorobutanesulfonate shows lower acute toxicity than the longer-chain PFOS and PFOA relatives, but questions remain about long-term bioaccumulation. Rodent studies have tracked blood clearance and metabolic breakdown rates, with some data suggesting limited persistence. Nonetheless, regulators flag all perfluorinated chemicals for caution, linked to ongoing concerns over water and soil mobility. Development teams routinely submit samples for in vitro and in vivo screening, with special attention to reproductive and developmental hazard endpoints. Measured environmental releases stay orders of magnitude below those seen in older surfactant industries, but waste labs maintain strict protocols for capture and incineration. Advocacy groups keep the pull toward transparency strong, asking suppliers and big end-users to disclose test results and support independent environmental health studies.
One thing stands out clearly: the future of dimethyl(phenyl)sulfanium perfluorobutanesulfonate rests on innovation and regulation. Researchers carry on fine-tuning derivative structures that keep the performance punch yet lower environmental persistence. More industries want “just enough” perfluorination to unlock technical gains without dragging along old PFAS baggage. High hopes ride on survivors in this class, able to balance safety, shelf life, and regulatory compliance. Vendors continue rolling out improved grades with tighter specs and enhanced data sheets, matching the demands of safety-conscious energy, water, and electronics sectors. Stakeholders in academia, industry, and non-profit groups push for shared repositories of best practices and benchmarks for toxicity and fate in natural systems. In my own experience, real progress gets measured not just in breakthroughs but in inch-by-inch improvements to synthetic methods, waste capture, and transparency with data. The promise of this sulfonium salt class answers as much to public trust and stewardship as to robust performance in tough technical niches.
Most people don’t come across names like Dimethyl(Phenyl)Sulfanium Perfluorobutanesulfonate in daily life. In chemistry circles, though, this compound has earned a reputation for punching above its weight. You’ll spot it when labs are doing the kind of work where the end product often defines how well electronics function. Chemists and process engineers have put it to use because of how reliably it can pass on sulfonium-based chemistry right onto a surface or within polymer reactions.
This tongue-twister of a molecule serves a very specific purpose: it acts as a photoacid generator (PAG). In every smartphone, tablet, or computer, thin slices of silicon—microchips—sit at their core. To etch the fine details onto those chips, manufacturers use photolithography. Here’s where Dimethyl(Phenyl)Sulfanium Perfluorobutanesulfonate steps in. Shining a beam of ultraviolet light hits the compound, triggering it to release acid, which then kickstarts a chemical reaction in a light-sensitive coating (the photoresist). Suddenly, a manufacturer can carve features into silicon that are narrower than a strand of hair. No reliable PAG, no fine features, no global tech industry as we know it.
Older PAGs released small acids, but their instability often turned into a big headache, leading to blurry edges on chips and missed lines in circuitry. Researchers sought out compounds that could hold steady through all the handling and pre-bake steps, but still let loose their acid right on cue—under light. Sulfanium salts like Dimethyl(Phenyl)Sulfanium Perfluorobutanesulfonate checked those boxes. With more control over acid release, chip lines got sharper, and yields went up. The compound’s perfluorobutanesulfonate group also brings solid solubility and chemical resistance, so harsh solvents and developers don’t break down the structure midway.
These advantages don’t come without worry. Fluorinated chemicals have made headlines over groundwater contamination and persistence in the environment. While Dimethyl(Phenyl)Sulfanium Perfluorobutanesulfonate works well in strict cleanroom settings, companies have started asking whether the performance justifies potential long-term impacts. Perfluorinated chains don’t degrade under sunlight or microbial action, so traces from manufacturing plants can hang around for decades.
I’ve talked with engineers in the semiconductor world who say it’s tough to swap out reliable PAGs overnight. Transitioning to new chemistry can take years—redesigning recipes, checking compatibility, running accelerated lifetime testing. Multinational firms have cranked up research for alternatives without the persistent fluorinated tail, but scaling up is slow.
A handful of companies have started piloting safer salt derivatives or blends with biodegradable acid sources. More work remains before these reach the same precision as current photoacid generators. Clear laws around chemical disclosures and robust wastewater treatment at semiconductor plants can help, but the technology gap isn’t closed yet.
The demand for smaller, faster chips isn’t dropping. Every step closer to a less persistent, more benign photoacid generator matters not just for the bottom line but also for the health of communities living around production hubs. Chemists, policy makers, and tech brands can drive solutions—nobody can go it alone on fixing the problem. The road from lab bench to full production runs takes patience and stubbornness, but past progress in this field shows that smart chemistry and clear rules make a difference.
Chemicals hold a strange place in our lives. Most people don’t spend much time thinking about them, until something leaks, catches fire, or sets off a stench throughout a warehouse. Years in both the lab and on the manufacturing floor taught me one thing: lax storage and sloppy handling turn minor problems into workplace emergencies. Chemicals don’t just misbehave on their own; people make choices that give trouble a chance to grow.
Some chemicals have a short fuse when exposed to heat or sunlight. Take hydrogen peroxide, for example. In an open-top bottle left under fluorescent lights, it can lose strength fast. Flammable liquids like acetone or ethanol don’t mix well with high temperatures. The flashpoint—the temperature at which fumes catch fire—often sits lower than room temperature, so a hot warehouse invites disaster. Tight-fitting containers and cooler, shaded rooms cut the risk. Anyone who’s popped open a swollen drum after a warm weekend learns this lesson quickly.
I've seen cases where a missing label on a drum triggers a whole chain of avoidable events, from needless lab tests to costly disposal. Chemical manufacturers don’t put storage guidelines on labels for fun. They expect anyone handling these products to follow those instructions to the letter. Failure here can mean toxic fumes, ruined batches, or contaminated equipment. For instance, acids stored near bases lead to violent reactions—sometimes even explosions. Split up incompatible materials. Check the Safety Data Sheet before new shipments arrive. Reading isn’t just for school kids; it’s for staying safe.
Splash goggles and gloves aren’t fashion statements, but they beat chemical burns. I once watched a co-worker mop up a mercury spill with bare hands, thinking it looked no worse than spilled water. He had to take a week off for testing and treatment. The basics—gloves, face shields, aprons—stop minor accidents from turning serious. If something smells strange or burns your eyes, fresh air should be the top priority. Having clear escape routes and ventilated storage rooms means nobody gets trapped by their own mistakes.
Routine checks matter. A forgotten shelf of old peroxides grows crystals that can blow up with the slightest touch. Secure locking cabinets stop unauthorized people from grabbing chemicals out of curiosity. Regular training and accountability make safe habits stick. Peer pressure works both ways: one careless worker creates risks for the whole crew, but a culture that calls out unsafe acts keeps everyone alert.
No fancy ventilation system or fireproof drum will save the day if the basics get ignored. Building a routine around education and open communication cuts accidents across the board. The best companies fund proper signage, pay for training, and treat every leak or spill as a red flag. For me, it boils down to vigilance and respect—for coworkers, for equipment, and for the chemicals themselves.
Dimethyl(phenyl)sulfanium perfluorobutanesulfonate might roll off few tongues, but this compound finds its way into niche industries, mainly involved with electronics and specialty chemistry. Seeing “perfluorobutanesulfonate” in its name sounds a warning for many who follow chemical safety. Perfluorinated substances fall into a family of chemicals that draw attention from both regulators and environmental researchers.
My own background in laboratory research and work with chemical inventories taught me one thing—if a compound features highly fluorinated chains, someone nearby is worrying about persistence. Perfluorinated chemicals, known for resisting breakdown, often linger in water, air, and soil for years. Once released, they refuse to simply disappear. They pop up in unexpected places, including drinking water far from any chemical plant.
Perfluorobutanesulfonate itself, often abbreviated as PFBS, replaced some notorious “forever chemicals” after stricter rules started phasing out older compounds like PFOS and PFOA. PFBS claims a shorter carbon chain, hinting at lower bioaccumulation and possible lower toxicity. Researchers, including teams at the US EPA and European Food Safety Authority, stress that “lower” does not equal “safe.” Published studies show PFBS can persist in the natural world longer than most organic contaminants, and animal studies point to problems with thyroid function and kidney effects.
Community members with experience working in manufacturing know the routine: new chemicals often replace hazardous ones, promising fewer risks. But occupational exposure to dimethyl(phenyl)sulfanium perfluorobutanesulfonate may bring health questions their replacements can't solve. Inhalation, skin contact, or even trace contamination from leaky storage can introduce risk. The trouble is, toxicological data for less common chemicals doesn't always arrive fast. The limited available information points to possible irritation, and animal studies from agencies like the National Toxicology Program show health impacts might show up long after exposure takes place.
Folks who fish or garden near industrial areas sometimes talk about the odd coincidence of unexpected health issues or loss of wildlife. PFBS and related chemicals don't break down easily, so they end up in surface water and groundwater. Plants take up these compounds, which then climb through food webs. Because regulators have moved away from some perfluorinated substances, tracking new chemicals poses real challenges in both testing and enforcement. Chemical companies tweak formulas just as fast as rules evolve, creating a game of regulatory whack-a-mole.
People deserve chemicals that don’t threaten their water, food, or bodies. One solution looks simple: start with transparency. Manufacturers should publish full toxicity reports and environmental fate studies before launching products. Communities can speak up for independent monitoring and support local water testing programs. Many places across the world now invest in advanced filtration—like activated carbon and ion exchange resins—specifically to grab these tricky compounds from drinking water. The cost comes out high, but so does ignoring the problem.
Most ordinary people don’t read chemical names for fun, but we all share the outcome. Pressure from regular folks combined with the latest science urges safer design, careful monitoring, and honest conversations about what lands in our environment. Each new compound deserves a full measure of caution until proven better.
As someone who spent years troubleshooting unexpected chemical reactions in both the classroom and the factory, I know most folks overlook stability until something goes wrong. Stability isn't about a molecule simply "sticking together." It's about how the substance acts in the real world—how it holds up on a warehouse shelf through a hot summer, in a cold truck trailer, or near an open bottle of bleach. Every bottle of product, every safety protocol, and every bit of wasted money ties back to whether that compound breaks down or hangs tough under stress.
Chemical stability matters every time a new process gets approved, a shipment is made, or a worker opens a drum. Most people remember ammonium nitrate in headlines, not for boosting wheat, but for its catastrophic explosions. Even something as simple as hydrogen peroxide can ruin an expensive batch of medical devices if improperly stored. If your compound crumbles in sunlight, sours in humidity, or transforms with a temperature spike, the risks snowball fast—sometimes right into disaster or regulatory fines.
Reactivity plays out like a bad prank. That stable-looking powder could suddenly ignite dust in a grain silo. A fancy additive could eat through stainless steel pipes after a year, long after anyone expects a problem. Chemistry isn't only about subtraction or addition; it can be about unwanted side adventures—side reactions that ruin product purity, shorten shelf life, or generate toxic fumes.
I've helped track down the culprit in more than a few ruined batches. One time, a stable-looking salt started behaving like a ghost ingredient, causing solid lumps in a liquid product. The real problem? Exposure to just a little bit of moisture in our warehouse shelves triggered ongoing, subtle changes—enough to gum up filling machines weeks later. Knowing these risks ahead of time—reading the safety data sheets, running shelf-life trials, poking at storage guidelines—helps keep things predictable.
Nobody should assume a compound will behave based on paper alone. Real-world chemical stability is proven through testing: heating, cooling, shaking, exposing to air, mixing with acids and bases, or even just waiting to see what grows inside a closed container. Quality control teams in pharma and food manufacturing don’t just cross their fingers; they push products to their limits to see how they’ll react to every curve ball. Catching instability early means safer products, fewer recalls, and less chaos for the people depending on them.
Science doesn’t solve these problems by wishing—solutions come from a careful blend of knowledge and vigilance. That means keeping formulas simple where possible, enforcing strict storage rules, and not skimping on environmental controls. It takes honest data-sharing with suppliers about purity and contamination, since even tiny impurities can jolt a stable compound into chaos. In the end, chemical stability and reactivity rest on the real-world handling, not just theory or tradition. A safe, reliable product reflects every choice—from the lab bench to the warehouse to the customer’s hands.
A spill catches people off guard. I’ve worked in settings where a simple oversight left chemicals on the floor, and in that instant, training moved from theory to reality. Panic never helps. Quick thinking and knowing what’s in front of you matter more.
People sometimes rush to grab a mop or towel without checking labels or material safety data sheets. Years ago, I watched a coworker get a splash of cleaner on his arm, ignore it for hours, and then regret it when the rash flared up. Recognizing signs of irritation or inhalation problems early stops a headache from turning into a hospital trip. Some folks shrug off mild symptoms, but stronger solutions or volatile vapors can have hidden effects.
Most spills don’t demand hazmat suits, but every workplace should keep gloves, absorbent pads, and eyewash stations close by. Even in my home garage, I stash baking soda for acids, and kitty litter for oil. I learned quickly that grabbing the closest rag spreads more than it soaks up. Clean-up starts with keeping the mess from growing. Barriers or simple sandbags can keep liquids from spreading under shelves, reaching drains, or soaking into carpet.
No one likes admitting mistakes, but silence puts others at risk. Once, a coworker tried to keep quiet about a solvent spill, and a week later, the stain led to a small fire. Talking up, no matter how embarrassed you feel, keeps everyone safer.Regular reminders and posters in plain sight reinforce how to act in an emergency. In places I’ve worked, quick huddles after a close call helped the team learn ways to do better next time. Stories about real incidents stick far better than dry training videos.
Safe handling isn’t only about reacting. Regular drills, reviewed every few months, pay off. Reading the label every time, not just during safety week, makes a habit. Keeping chemicals in their labeled containers and on the right shelf keeps confusion low and response times short during chaos.Building a checklist for storage and waste disposal keeps trouble from building up. A friend once left open cans carelessly stacked in a corner. One tipped, fumes spread, and it cut everyone’s workday short. Simple routines stop drama before it starts.
A good response to exposure relies on teamwork. Someone needs to man the phones, another needs to assist the exposed coworker, and another should guide help to the right spot. In my first warehouse job, we made mistakes because everyone rushed to help alone. After learning from that scramble, we set clear roles and saved precious time the next time a scare happened.
After every cleanup, reviewing the sequence seriously cuts down on repeats. Companies and families alike can benefit from logging incidents and improvements. Safety experts say most injuries could be avoided by learning from the last oops. Process over perfection always wins.
Having the right supplies well-stocked, reading labels, acting fast, and keeping communication open all turn small spills into little lessons, not disasters. We don’t control accidents, but the way we respond sets the tone for everyone’s health and confidence on the job and at home.
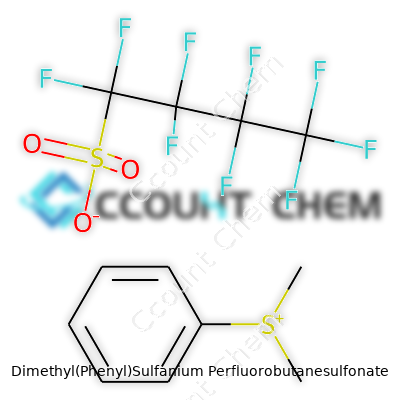
| Names | |
| Preferred IUPAC name | Dimethyl(phenyl)sulfanium nonafluorobutane-1-sulfonate |
| Other names |
Perfluorobutanesulfonic acid dimethyl(phenyl)sulfonium salt Dimethylphenylsulfonium perfluorobutanesulfonate Sulfonium, dimethyl(phenyl)-, perfluorobutanesulfonate |
| Pronunciation | /daɪˈmɛθɪl ˈfiːnɪl sʌlˈfeɪniəm ˌpɜːrˌflʊəroʊˌbjuːteɪnˈsʌlˌfəˌneɪt/ |
| Identifiers | |
| CAS Number | 79981-30-7 |
| 3D model (JSmol) | `8AAQSkFBAAAABAAADwABAAAAAAAcAAIAAgAADAAGAAAAFAAAAAEAAAAmAAAAAQAAABAAAAAA4gAAACsqKhISEkA6CgAAACslJSUzQT0zQGRENDI0JDYsLCspLyglLyQ1LiEQExAJCQcIAAAICD81RCQWJCQuKyYjLCEuNDAgNBUlJUMWLCkvNTI8TkRVR0c2PSklYVBEJDwtQCooLDIkNzMxNycsJTMbGhyoRSslAA==` |
| Beilstein Reference | 3918736 |
| ChEBI | CHEBI:139748 |
| ChEMBL | CHEMBL4447075 |
| ChemSpider | 28644165 |
| DrugBank | DB11258 |
| ECHA InfoCard | 100.211.097 |
| EC Number | 80922-20-1 |
| Gmelin Reference | Gmelin Reference: 2076225 |
| KEGG | C22107375 |
| MeSH | D013902 |
| PubChem CID | 162197251 |
| RTECS number | GV8888000 |
| UNII | E3G3T9K86H |
| UN number | UN3274 |
| Properties | |
| Chemical formula | C10H13F9O3S2 |
| Molar mass | 380.31 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.51 g/cm³ |
| Solubility in water | insoluble |
| log P | -2.15 |
| Acidity (pKa) | -3.7 |
| Basicity (pKb) | -2.3 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.481 |
| Viscosity | 0.982 cP |
| Dipole moment | 4.05 D |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H317, H319 |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313, P501 |
| LD50 (median dose) | LD₅₀ (oral, rat): >2000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Dimethyl(phenyl)sulfonium triflate Dimethyl(phenyl)sulfonium tetrafluoroborate Dimethyl(phenyl)sulfonium hexafluorophosphate Dimethyl(phenyl)sulfonium chloride Dimethyl(phenyl)sulfonium bromide |