Diguanidinium carbonate barely made a whisper in early chemical catalogs, yet once its value for electronics and specialty materials became clear, researchers put their backs into developing scalable methods. Academic publications in the late twentieth century started digging into the molecule’s guanidine backbone, unlocking new directions for organic synthesis and ionic compound research. Industrial uptake tracked with the emerging need for highly pure intermediates in digital displays and specialty polymers. As the digital boom raised the bar for both purity and performance, the search for efficient, reproducible synthesis methods picked up pace. Patent filings from the 1990s through today reveal a steady arm wrestle between yield, impurity profile, and product stability. Today, leading suppliers can trace their methods to research groups and pilot plants that kept fine-tuning parameters to better serve semiconductor, agriculture, and pharmaceutical clients.
Diguanidinium carbonate stands out as a strong organic base, bringing guanidinium’s distinctive basicity into a stable, crystalline salt. The material packs a punch as a reagent, catalyst precursor, and buffer. Lab techs know it best for controlling pH in sensitive polymer and coating reactions. Beyond the lab, factories use it to craft safer, more efficient chemical transformations, giving rise to subtle shifts in surface chemistry or product lifespan. This compound comes sealed in high-density polyethylene drums or double-lined bags to put a lid on moisture, since guanidinium salts pull in water vapor from even mildly humid air.
Crystalline diguanidinium carbonate usually looks like a white to off-white powder. Expect it to dissolve easily in water, where it forms a strongly alkaline solution. The molecular weight hovers around 238 grams per mole, and it keeps a melting point well above 150 degrees Celsius, holding its shape in all but the hottest conditions. No strong odors signal its presence, helpful for workers handling open vats. Guanidinium ions square off against carbonate groups to reach a stable, neutral salt, but extreme acids and bases break this bond down quickly. Stability against mild heat and air lets it thrive in industrial and research warehouses, though strong oxidizers chip away at the structure over time.
Chemical buyers like to see at least 99% purity for demanding research and electronic applications, so reputable producers certify both guanidinium and carbonate content by titration or chromatography. Top suppliers ship with detailed certificates of analysis listing moisture, free base, and heavy metal traces. Labeling always includes the UN number, recommended handling and storage conditions, and batch traceability codes. Product safety data sheets flag inhalation and skin contact risks, along with fire and spill procedures. Facilities running digital or automated inventory stick barcodes to every drum for rigid compliance with chemical tracking regulations.
The mainstream preparation process brings guanidine salts together with alkali carbonates in aqueous solution. Operators run this reaction in stainless steel reactors at slightly elevated temperature while stirring to prevent hot spots. Carbon dioxide bubbles off as diguanidinium carbonate forms and drops out of solution. A round of filtration grabs the target molecule, and rotary evaporation dries the white crystals under vacuum. Some operators add further purification steps – like recrystallization – to drive off trace amine or salt byproducts and tighten the purity window. Plant managers monitor pH and conductivity right up to the drying stage, dialing in the balance between throughput and product quality.
Chemists prize diguanidinium carbonate as a versatile building block. The strong guanidine groups give a hefty push in deprotonation and condensation reactions, tasty for organic synthesis and new catalyst structures. The carbonate can release carbon dioxide in acidic media or as temperatures rise, opening the way to gentle, gas-evolving chemistry. Research groups push the molecule into derivative forms – swapping anions or grafting onto polymers – to change solubility, reactivity, or thermal stability for next-generation battery materials or custom agrochemicals. Some industries use the compound to pull off selective extractions or crystallizations, taking advantage of its unique charge balance and hydrogen-bonding patterns.
Diguanidinium carbonate travels under a handful of names: bisguanidinium carbonate, guanidine carbonate (when shorthand is tolerated), or its IUPAC label, 1,1'-carbonyldiiminourea. Trade names pop up depending on supplier and target market, with proprietary sub-brands focusing on ultra-pure, electronic-grade, or reactive forms. Catalogs list CAS number 593-85-1 alongside synonyms for ease of sourcing. To keep things straight, lab staff check both structural formula and supplier certificate, avoiding mix-ups with the simpler monoguanidinium carbonate or unrelated guanidinium organics.
Handling this chemical means paying special attention to skin and eye protection, since dust or concentrated solutions can cause burns. Long sleeves, safety goggles, and chemical-resistant gloves reduce risk. Inhalation hazards remain low under normal ventilation, but spill response teams use particulate respirators and chemical aprons for cleanup. OSHA and REACH agencies push for documented risk assessments, hazard communication, and incident reporting. Spill control plans include dedicated neutralization stations and labeled containers, with regular audits to check for compliance. Environmental controls collect rinse water and any waste, routing it to treatment rather than general drains, since guanidine-derived compounds break down slowly in surface water.
This compound steps up in fields demanding precise bases and buffering agents. Electronics and optics manufacturers rely on bulk shipments to control deposition chemistry during thin-film device production. High-end catalyst developers lean on its basic strength and handling ease for metal-organic frameworks and transition-metal catalysts. Pharmaceutical companies string it into multi-step syntheses, exploiting the predictable reactivity and aqueous solubility. In agriculture, specialists test modified forms as nitrogen-release agents with slow-leaching behavior. Battery researchers grab diguanidinium carbonate when hunting for new electrolytes or ionic conductors, as guanidine groups play nice with a wide variety of ion pairs.
Academic and corporate labs push research on diguanidinium carbonate in three main streams: synthetic routes, derivative materials, and performance in real-world applications. Scientists continue searching for greener ways to make the salt, such as enzyme-assisted or solvent-free routes that skip harsh starting materials. Material scientists combine it with new co-monomers or metallic additives, building up custom matrices for sensors, fuel cells, or high-end insulation foam. Data pours in on reactivity with inorganic and organic partners, feeding machine learning projects predicting new catalyst or pharmaceutical targets. Many teams upload their latest findings to open-access databases, giving a clear shot of what works and what hits a dead end.
Toxicologists look at both acute exposure risk and long-range health effects. Early animal studies show low oral toxicity at expected workplace exposures but suggest skin and eye tissue damage after direct contact with concentrated compound. Repeated dosing over weeks in rodents led to minor liver enzyme changes, suggesting long-term occupational exposures call for ongoing health monitoring. Environmental scientists track breakdown products in soil and water, flagging slow degradation compared to simple ammonium or urea compounds. Lab studies show the compound binds weakly to soil particles and does not bioaccumulate in fish or plants, but authorities stress the need for further outdoor testing to confirm these patterns in the wild. Regulators urge continued research on reproductive and developmental effects, as guanidinium derivatives show mixed signals depending on dose and exposure route.
Future demand for diguanidinium carbonate ties tightly to trends in electronics, high-performance materials, and sustainable agriculture. Companies scaling up advanced displays, flexible solar panels, and next-gen batteries will stretch producer capacity for both quantity and purity—pushing for more energy-efficient synthesis and smarter purification processes. Regulatory changes around solvent use and water conservation force R&D teams to skip legacy chemistry in favor of lower-impact manufacturing. Chemists race to bolt on new chemical groups or make diguanidinium carbonate fit recycling and closed-loop systems, instead of single-use or landfill outcomes. Tracking new patents, research conferences, and supplier advances gives clear signals that diguanidinium carbonate, once a lab oddity, now sits firmly in the toolkit for tomorrow’s electronics, clean tech, and custom chemical stories.
Tucked away in the shelves of technical supply houses, diguanidinium carbonate rarely appears in consumer headlines, but this chemical supports a surprising number of industries. Its main value starts with its chemical structure, which brings two guanidine groups together through a carbonate bridge. This unique configuration gives it more than just a stable shelf life—it allows the substance to behave in ways other compounds can’t match.
Anyone working in specialty chemicals or industrial applications will notice how diguanidinium carbonate gets paired with other substances to modify performance. As an intermediate, it gets called on in the development of flame retardants. Fire safety standards rely on compounds like this. Once blended with phosphorus-based chemicals, it helps curtail spread and lowers the flammability rating of plastics, foams, and textiles. Lawmakers started demanding these measures as electrical equipment and synthetic materials made their way into public buildings, offices, and airplanes.
Drug development teams have also learned to value this compound. Certain pharmaceutical processes adopt diguanidinium carbonate as a building block for more complex molecules, especially where the inclusion of guanidine groups ensures stability or desired solubility. Production runs for niche drugs, especially those requiring custom heterocycles, sometimes depend on it. The end users—patients hoping for a better or less toxic therapeutic option—won’t see diguanidinium carbonate on a label, but it keeps progress possible along the way.
Concerns always appear once specialty chemicals leave the lab and enter manufacturing. Some compounds linked to flame retardancy have drawn sharp criticism for environmental persistence or possible toxicity. Diguanidinium carbonate enters this discussion, but right now, it isn’t seen in the same risky category as older halogenated compounds. Guidelines published by regulatory agencies don’t flag this substance as especially hazardous, but practical experience on the shop floor always makes me double-check my safety datasheets before using anything new. That’s just good practice, especially with any guanidine-based substance, and I make sure personal protective equipment is more than an afterthought.
Waste management needs a closer eye across many chemical plants. Most companies treat wastewater and chemical leftovers on-site before discharge. I’ve noticed a trend toward investing in recycling and reclaiming chemical byproducts, not just from legal pressure but from a cost-saving point of view. As rules tighten, those industries depending on diguanidinium carbonate and other intermediates should keep testing their disposal methods and seeking cleaner synthesis routes.
Supply chains now expect responsible sourcing, transparent labeling, and new answers when regulators ask how each intermediate impacts the final product. Chemical engineers keep searching for safer and more efficient synthesis—an ongoing process since every batch means new variables and, sometimes, new challenges. Using diguanidinium carbonate wisely starts long before purchase: knowing why it gets picked, storing it properly on site, protecting workers, and avoiding unnecessary waste.
Better risk assessment brings peace of mind for customers and workers alike. Each innovation in formulations or fire resistance, each improvement in pharmaceutical synthesis, owes something to intermediates like diguanidinium carbonate. For consumers, the most important part remains trust that the products and medicines reaching them owe as much to care in handling as to clever chemistry.
Diguanidinium carbonate stands out in the world of specialty chemicals. The chemical formula for diguanidinium carbonate is C3H14N8O3. The structure itself starts by bringing together two guanidinium ions with a single carbonate ion. Anyone who's ever spent time mixing solutions in a lab or checking ingredient lists for industrial compounds has seen patterns like this. Two cations grab onto an anion, balancing charge and forming a salt that's both stable and useful.
Let’s talk composition. Guanidine has the formula CH5N3. As a cation, guanidinium gives up an electron, so the formula stays the same but picks up a positive charge: [C(NH2)3]+. Grab two of them, add CO32– (carbonate), and suddenly you’re dealing with two guanidinium ions pairing with the carbonate to give you the answer: (C(NH2)3)2CO3. Or if you count everything out, you hit the C3H14N8O3 count. This formula shows a real connection between the organic side—loaded with nitrogen—and the inorganic carbonate.
My own chemistry background taught me that knowing the formula is more than memorizing numbers and letters. The formula tells you how that compound behaves in water, what kinds of chemical reactions you can run, even how you can store or transport it without trouble. For research, the exact stoichiometry reveals if unexpected side reactions crop up. Labs running enzyme studies or people tuning performance chemicals depend on this accuracy. You get an idea not just about safety, but about costs—right down to the scale at which it can be made in bulk.
Diguanidinium carbonate doesn’t turn up often in textbooks, yet folks using it in synthesis or specialty production pay close attention to its formula because it spells out its reactivity. As with a lot of nitrogen-rich compounds, storage in dry and cool conditions is key. Small bits of moisture or contamination can cause the chemical to behave unpredictably, so clear labeling, good training, and reliable data sheets help prevent accidents or wasted time. Every lab tech and production manager I know wants that peace of mind before opening any unlabeled jar.
Problems crop up when formulas slip through the cracks or get copied wrong from supplier sheets. Mistakes here mess with experimental outcomes, and sometimes even lead to dangerous surprises. Sticking to verified chemical databases or trusted suppliers protects not just workers, but downstream uses. QR codes tied to digital certificates now track both purity and proper structure, cutting down on risk and confusion. Teaching students the importance of chemical notation gives them the skills to spot problems before they leave the bench.
Diguanidinium carbonate serves as a good example for anyone looking to understand the importance of chemical literacy in both industry and research. Transparent sharing of chemical formulas empowers safe, rigorous innovation. As I’ve learned from lab work and consulting—trust starts with getting the basics right, so the whole chain of use stays solid.
Diguanidinium carbonate does not turn up in everyday conversations, but it’s not an obscure compound in laboratories. I first worked with it in a research setting that valued safety. Before lifting the lid on any chemical container, we dug into everything from the molecular structure to human health data. It’s not just because of policy—self-preservation truly kicks in when you realize how unpredictable chemicals can be.
Most folks can’t pronounce diguanidinium carbonate, and honestly, nobody blames them. The reasons for handling it with care lie in the data. According to the European Chemicals Agency (ECHA), diguanidinium carbonate is not flagged as a carcinogen, reproductive toxicant, or mutagen. It isn’t on any list for persistent bioaccumulative toxic substances. Yet, the absence of red flags doesn’t mean nobody should worry. Exposure routes—skin, inhalation, or ingestion—matter with any chemical. On paper, diguanidinium carbonate does not create the type of panic seen with stuff like mercury or benzene, but sensible precautions always make sense.
From what I’ve experienced, dust can trigger mild respiratory irritation for sensitive people. It can also cause a bit of skin and eye irritation. No surprise—lots of inorganic salts do. During the grad school years, gloves and goggles showed their value, even when things seemed “safe.” Official safety data sheets confirm what instinct told us: avoid breathing in the powder and don’t let it linger on bare skin.
In a lab, nothing gets taken for granted. Splash goggles, gloves, well-ventilated workspaces—the rules matter, no matter how many times you’ve opened that bottle before. In industries where diguanidinium carbonate is part of the process, these habits stay in place for a reason. Mistakes rarely happen when everyone’s trained and protocols get followed. Some workplaces run into higher risks because of poor housekeeping or cutting corners when nobody is looking.
Scientists care about what happens after a chemical is used. From the data, diguanidinium carbonate doesn’t break down in ways that fill waterways with toxic runoff. It’s not been tagged as a major threat to aquatic life by the EPA or similar agencies. Personally, seeing this compound handled in effluent systems reassured me—a step above dumping it down the drain, even if it isn’t classified among the real “bad actors” of water pollution.
Reading material safety data, I see the same recommendations across the board: minimize dust, use gloves, keep emergency eyewash in reach. Labeling and storage require following the book. Safety isn’t a one-time checklist. During my years spent wandering between academic labs and commercial facilities, nobody regretted spending an extra minute double-bagging a waste container or scrubbing down benches. In fact, that’s where I realized what separates a minor irritation from a costly incident.
People often tune out the safety talk after a while, convinced another person will clean up the mess. One answer is regular, no-nonsense training—make the rules practical, get workers involved, and update protocols as new data appear. Accidents can sneak in at the margins, not during headline-grabbing moments. In my experience, making risk information visual—charts, simple labels, real stories—sticks better than another memorized lecture. For diguanidinium carbonate, a straightforward approach beats complacency. Responsibility doesn’t cost much, but it pays off every single time.
Anyone responsible for handling chemicals quickly learns the value of good storage habits. Diguanidinium carbonate brings its own set of rules. This compound won’t tolerate shortcuts. I’ve seen talented lab techs lose entire stockpiles to careless humidity, and it’s frustrating to witness resources and time go down the drain just because one small corner of a shelf missed standard protocols.
Diguanidinium carbonate attracts water like a magnet. The presence of excess humidity can cause it to clump, degrade, or even react. Direct exposure to air makes that process happen faster, and the powder’s consistency shifts in no time. Storing this compound in tightly sealed containers within a dry cabinet makes all the difference. Adding a silica gel packet inside containers offers a cheap and reliable barrier. Checking seals after every use helps avoid unpleasant surprises.
Unstable temperatures threaten more than comfort, especially with chemicals. Diguanidinium carbonate handles room temperature, but heat can push it beyond safety. Shelves next to radiators or sunny windows become traps. Keeping storage areas cool, shaded, and well-ventilated stands as the baseline. Refrigerators create problems by introducing condensation if chemicals warm up after removal. No one wants to see their materials degrade because of a few degrees.
Mislabeling starts small—someone borrows a scoop, and the lid goes back with a handwritten note, or no note at all. Eventually, confusion clouds everything. I witnessed a storage room get emptied in a recall after a labeling mix-up caused a scare. Every container must carry the name, date received, and condition. Digital inventory helps, but a simple marker on every jar works wonders. You can prevent accidental misuse or mixing with incompatible materials just by double-checking the label.
Combining chemicals sounds risky, and it becomes dangerous if you skip separation. Diguanidinium carbonate needs its own shelf—away from acids, oxidizers, and food items. Flammable substances and common household cleaners should never share space. Lab managers enforce clear colour-coding and storage diagrams for a reason. Even with years of experience, I trust a chart taped to the wall more than memory during a busy shift. Routine audits guarantee that mistakes don’t become patterns.
Storing diguanidinium carbonate means planning for both immediate use and longevity. Inventory checks every six months help spot signs of deterioration—powder caking, discoloration, or odd smells indicate issues. Disposing of old stock properly signals a respect for workplace safety and environmental health. I’ve learned that regular deep cleaning and inspections make a dramatic impact—reducing waste and keeping everyone safer. Involve the team in safety reviews, and storage stops feeling like a chore.
Safe storage builds trust, saves money, and, most importantly, protects people. Setting up best practices for chemicals like diguanidinium carbonate isn’t a one-time deal. Training repeats, containers get checked, and storage spaces adapt to new guidelines. Whenever someone asks me about safe practices, I bring up specific examples and lessons learned the hard way. No detail is too small. The final step comes from a simple promise: Treat every material as something valuable—never cut corners, never get lazy, and always check twice.
Diguanidinium carbonate belongs to the class of guanidinium compounds, often used in specialty chemicals and sometimes research labs. It looks like a white powder—nothing flashy, but the substance can cause trouble if treated carelessly. I have spent enough time around chemical stocks to know how things can go wrong with substances that look pretty harmless on a shelf.
Diguanidinium carbonate may not grab headlines like lead or mercury, but exposure can hurt you. Direct skin contact or a whiff of the dust can cause irritation. No one enjoys a rash or a burner in the nostrils. I always reach for latex or nitrile gloves, a decent lab coat, and snug goggles when the bottle comes out. A dust mask or, better, a certified respirator keeps airborne particles out of your lungs. Small steps like wearing gloves and a mask sound routine, but I’ve seen seasoned pros complain for days after skipping this gear.
Working with dry, powdered chemicals ramps up dust risk. Opening Diguanidinium carbonate containers under a fume hood saves you plenty of pain. If a fume hood isn’t handy, crack a window and run an exhaust fan—just keep dust from hanging around. The point isn’t to act tough, but to avoid headaches from breathing something you can’t even see. I’ve learned that you don’t have to see danger for it to land you in the ER.
Moisture breaks this compound down—leave the lid off and you’ll get a clumpy mess. It pays to keep Diguanidinium carbonate in a tightly closed bottle, stored in a dry place, away from acids. I stick with high shelves to keep it out of reach, and I label everything clearly. After seeing a colleague confuse bottles once—fortunately with water, not acid—I know clear labels and careful placement really matter.
Everybody spills things—thinking it won’t ever happen sets you up for real trouble. If powder lands on your bench, sweep it up using a brush and dustpan designed for chemicals. Avoid vacuuming unless your vacuum bears a HEPA filter built for hazardous dust. I always mop up the remnants with a damp cloth, bag it all, and toss it in hazardous chemical waste. Dumping it in the regular trash isn’t just sloppy, it breaks the law in dozens of places.
I’ve noticed newcomers try to cut corners on safety protocol thinking Diguanidinium carbonate can’t be that dangerous. Any chemical with warnings on the label deserves respect. Training keeps people from skipping critical steps. As for disposal, this chemical doesn’t go down the sink or in municipal trash. Lab managers or environmental health and safety officers offer guidance on correct procedures—ignoring them invites fines, and worse, accidents.
Safe chemical handling starts with old-fashioned care and a little humility. No one expects a white powder to bite back, so it’s easy to get careless. Creating routines for gear, storage, clean-up, and disposal protects everyone. My time in labs taught me that you only need one mistake to cost someone a week of sick time, or more. Quick reminders and regular retraining make a huge difference, and nothing beats a culture where everyone reminds each other what safe practice looks like. Diguanidinium carbonate might not scare most folks, but respect for the basics always pays off.
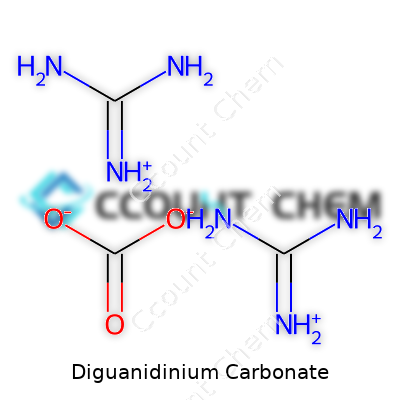
| Names | |
| Preferred IUPAC name | Diguanidinium carbonate |
| Other names |
Guanidine carbonate Carbonic acid diguanidine salt Diguanidinium carbonate |
| Pronunciation | /dɪˌɡwæn.ɪˈdɪn.i.əm ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 10550-13-3 |
| Beilstein Reference | 3561577 |
| ChEBI | CHEBI:91515 |
| ChEMBL | CHEMBL2106075 |
| ChemSpider | 22221 |
| DrugBank | DB13857 |
| ECHA InfoCard | 03beea27-8842-33c6-b8b4-2f42745f2c4a |
| EC Number | 208-618-6 |
| Gmelin Reference | 371801 |
| KEGG | C20905737 |
| MeSH | D003002 |
| PubChem CID | 23695698 |
| RTECS number | HE7175000 |
| UNII | 5Q1842JPGA |
| UN number | UN3249 |
| Properties | |
| Chemical formula | C3H12N8O3 |
| Molar mass | 237.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | **Soluble** |
| log P | -3.1 |
| Vapor pressure | < 0.01 mmHg (20 °C) |
| Acidity (pKa) | 8.2 |
| Basicity (pKb) | 1.48 |
| Magnetic susceptibility (χ) | -51.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.59 |
| Viscosity | Viscosity: 64.8 cP (25 °C) |
| Dipole moment | 6.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -318.0 kJ/mol |
| Pharmacology | |
| ATC code | A10BX03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | **GHS labelling of Diguanidinium Carbonate:** ``` GHS07, Warning, H315, H319, H335, P261, P264, P271, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 ``` |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2-8°C |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Guanidinium chloride Guanidinium thiocyanate Guanidinium sulfate Guanidinium nitrate |