Copper has played an enormous role in both industry and science. Its story leads through centuries of mining, metallurgy, and chemistry, tracing back to ancient times with simple salts and oxides. The march toward copper(II) methanesulfonate took shape much later, woven into the wider push for safer, more efficient copper compounds. In the late twentieth century, researchers and industry sought alternatives to the classic copper sulfate. Growing pressure for greener electroplating agents pushed attention to sulfonate complexes. Copper(II) methanesulfonate came into sharper focus because of its stability in water and relatively low environmental impact. I recall reading through technical papers from the 1990s where labs struggled to reduce hazardous waste from copper baths, and methanesulfonic acid offered straightforward, biodegradable sulfonate anions. Through trial, error, and steady refinement, this compound worked its way into mainstream applications.
Copper(II) methanesulfonate arrives mainly as a blue crystalline solid or as a concentrated aqueous solution. Both forms dissolve readily in water, producing an intense blue hue that betrays the copper(II) ion within. The compound often rides in drums or plastic jugs, labeled for lab, industrial, or electroplating use. Practical folks value its vivid color for quick identification and its low odor compared to other copper salts. This matters day-to-day, as anyone working with more pungent copper compounds—like nitrate or ammonia complexes—will quickly appreciate milder replacements. It sees action in circuit board manufacturing, metal finishing, and even organic synthesis. The price sits above the basic copper salts, reflecting added processing demands, yet users prize its performance and handling.
The anhydrous form of copper(II) methanesulfonate melts above 200°C, decomposing with release of sulfur dioxide and copper oxides. It weighs in with a molar mass near 245.7 g/mol, and forms thin, flat crystals that dissolve freely in water or dilute methanesulfonic acid, but stubbornly resist most simple organics. Its solution stays acidic. In the hands of a careful tech, its conductivity and stability allow nearly trouble-free copper deposition even at low concentrations. The compound avoids pesky byproducts common to sulfate-based baths, shifting the balance toward clean metal finishes. Measuring its solubility at different temperatures shows almost linear increases—fewer headaches for bath maintenance teams. Results from X-ray diffraction and IR spectroscopy confirm a classic octahedral copper(II) geometry, bound by two sulfonate oxygens and coordinated water.
Labels on copper(II) methanesulfonate drum readouts make or break a production line’s efficiency. Standard bottles list copper content, usually between 19-22% by mass, alongside trace contaminant numbers like lead or nickel, strict pH range, and the density of the stock solution. Labs and factories demand certificates of analysis with every batch—showing not just the compound’s purity, but the full rundown on potential impurities. Modern regulatory rules call for hazard pictograms, batch numbers, and safety statements. These labels matter because users have to track material flows, manage exposures, and answer to environmental audits. I’ve worked in shops where a missing specification on a drum label triggered a halt across a plating line—every gram must be traceable.
Making copper(II) methanesulfonate on an industrial scale usually starts with copper carbonate or oxide. Chemists treat these raw materials with methanesulfonic acid under controlled heating, stirring all the while to avoid hotspots and sludgy pockets. Once the reaction runs to completion, technicians filter the mixture to pull out insoluble filth, then concentrate solutions by evaporation under reduced pressure. The result is a blue-green syrup that crystallizes on cooling. Labs run purity checks by titration and spectroscopy. Any trace of greenish color or brown indicates slow contamination—process tweaks fix it quickly. On a small scale, students can try the synthesis in a fume hood, learning firsthand that making specialty copper salts requires patient addition and constant pH monitoring.
Copper(II) methanesulfonate stands apart for its stability and versatility. In the wilds of the chemistry lab, it can swap its sulfonate for other ligands, build up complex ions, or reduce to metallic copper on demand. Electroplating lines run electric current through solutions of this salt, pulling bright copper out onto metal surfaces. Organic chemists sometimes coax it into serving as a catalyst for oxidative couplings or cross-coupling reactions—methanesulfonate’s lone pair charge sheltering the copper core. Folks who tinker with high-end synthesis value the clean, non-fuming byproducts, avoiding the sulfuric smog and sludge of old-school copper salts. Copper(II) methanesulfonate also slips quietly into redox cycles, giving up and grabbing electrons as needed without giving the glassware a hard time.
Around the world, this compound walks under several names. Some call it simply copper methanesulfonate; others write copper(II) mesylate, copper(II) methanesulphate, or copper(II) methylsulfonate. Labels may use CAS numbers or supplier codes as shorthand. Common product codes from established chemical houses simplify repeat orders. Still, confusion crops up if catalog or shipment documentation uses older or regionally favored terms. For anyone running a stockroom or reordering for a busy process line, this naming maze can stall deliveries and muddy safety compliance. That’s why experienced hands always cross-check certificates and SDS pages before pouring a new drum into the tank.
Copper(II) methanesulfonate, though milder smelling than some salts, ranks as hazardous if spilled or handled carelessly. Skin and eye contact causes irritation—sometimes severe—and inhalation of dust or vapors should be avoided. Standard PPE includes gloves, goggles, and lab coats. Facilities require ventilation, spill kits, and eyewashes close to any work zone. Waste must be collected and disposed as per local rules, often as hazardous material due to copper’s aquatic toxicity. Safety Data Sheets remind users of incompatibility with strong reducing agents or alkalies. I’ve seen one mishap from spilled concentrate eat right through the paint on a bench, and another incident where a missed glove left a nasty red patch on the skin. Labeling must include pictograms, precautionary statements, and first-aid advice readable at a glance.
Copper(II) methanesulfonate wins favor in electronics manufacturing, especially for printed circuit board (PCB) plating. The methanesulfonic acid system, made possible by this salt, yields bright, even copper coatings without the sludge or sulfate crystals that plagued earlier formulas. Surface finishes on connectors or heat sinks benefit from its fast, controllable deposition. Small-scale battery research and lab syntheses appreciate reliable copper sources that don’t drag along dye contaminants or dense sludge. In the past decade, I’ve seen research papers exploring this compound’s use in microelectronics and nanostructure growth, exploiting predictable crystal habits. Environmental specialists point out that methanesulfonate-based systems produce less problematic waste, reducing overall chemical load on downstream water treatment facilities.
R&D teams tinker with copper(II) methanesulfonate to address both technical and green chemistry goals. Universities and big manufacturers team up to study new electrolyte additives for smoother copper films critical for fine-pitch circuits. Analytical chemists focus on unraveling impurity effects on surface grain size and adhesion. Several groups push for continuous-flow syntheses, cutting energy and water use. Other teams probe this copper salt’s behavior in electrocatalysis, hoping to unlock new reactions for green hydrogen production or CO2 reduction. As data piles up, specialists share findings at technical conferences, debating how best to keep costs in check without sacrificing product quality. My own experience with copper surface studies using methanesulfonate baths showed less nodulation and pitting than other formulations, improving both yield and downstream processing.
Toxicology stands front and center for any copper compound. Copper(II) methanesulfonate falls under the general umbrella of copper(II) salts, which are toxic to aquatic life and potentially irritating to human tissue. Chronic overexposure can damage kidney and liver function, and careful animal studies map out the limits of acceptable occupational exposure. Methanesulfonic acid, though biodegradable, still pushes acidity levels and needs careful neutralization before disposal. Agencies regulate effluent copper concentration tightly to protect riverine and marine ecosystems. Recent risk assessment studies run by independent labs confirm that while operator risk remains manageable with good practices, strict engineering controls stay necessary. Companies invest in closed systems, automated tank emptying, and frequent air monitoring to keep exposures safely below permissible limits.
Looking ahead, copper(II) methanesulfonate holds promise for greener and more precise forms of electrochemical manufacturing. Pushes for miniaturization in electronics, lower emissions in metal finishing, and recyclable electrolyte systems all favor compounds with clean reaction profiles and high solubility. Researchers experiment with slower-release formulations and hybrid metal baths to cut down copper losses. With regulatory and consumer attention tightening on every step of the supply chain, this compound’s low-smog, biodegradable sulfonate scaffold helps companies stay on the right side of new standards. Improved synthesis and purification could bring prices down, spreading its adoption from high-tech factories to mid-tier manufacturers around the world. Those charging ahead with copper-based catalysis spot opportunities in energy storage and environmental remediation, betting on copper(II) methanesulfonate as a dependable workhorse for the next phase of chemical innovation.
Copper(II) methanesulfonate rarely shows up in headlines, but in the electronics industry, it’s busy quietly powering production lines. If you’ve held a smartphone or a high-end printed circuit board, it’s likely copper(II) methanesulfonate had a role somewhere in the making. The compound offers strong solubility in water, meaning technicians can dissolve it fast and run smooth electroplating operations. This property supports even and consistent copper deposits onto various surfaces. That’s fundamental for electronics manufacturing, where even a tiny gap in a copper layer can spell trouble for performance or reliability.
Older forms of copper salts in plating use to involve harsher acids. Those processes produced more hazardous byproducts, making waste management more costly and risky. Copper(II) methanesulfonate, paired with methanesulfonic acid, takes a gentler approach. Its byproducts are less problematic for disposal, giving manufacturers a cleaner option and reducing daily headaches over regulatory compliance. As environmental rules get stricter, straightforward, safer options have real value. If a plant manager isn’t worrying about hazardous waste violations, they’re likely getting more sleep—and fewer unexpected fines.
Metal finishers count on this compound not just for high-tech jobs but also on classic applications. Decorative plating – from door handles to jewelry – gains brighter, more durable copper finishes through solutions using copper(II) methanesulfonate. The consistent results mean fewer rejects and less rework, which saves time and material. Battery researchers also pay attention. Recent studies show copper(II) methanesulfonate has potential for new battery chemistries, especially those focusing on sustainability and recycling. Copper deposition plays a direct role in battery electrode formation. A more reliable supply chain for copper chemicals could push battery developments further, making renewable energy storage more effective and affordable.
No chemical comes without tradeoffs. The cost of producing copper(II) methanesulfonate tends to run a bit higher than regular copper sulfate. Some small firms hesitate to make the switch because their old systems still do the job. Large factories with tighter environmental standards and a focus on worker safety have pushed forward, and their success sets a model for others. What tips the scales is often a mixture of regulatory pressure, long-term savings on cleanup, and a bigger market demand for eco-friendly practices.
As more tech products and batteries enter the global market, the industry’s gaze rests partly on cost and partly on reputation. Copper(II) methanesulfonate addresses both. Government incentives or subsidies for safer plating chemicals could help small and mid-tier firms transition. Research efforts to develop lower-cost production methods, or recycling from spent electrolytes, can also drop prices and make this option more attractive. The more manufacturers prioritize safety, product quality, and waste management, the more this compound stands to gain ground — making products just a bit greener and a touch more reliable with every switch.
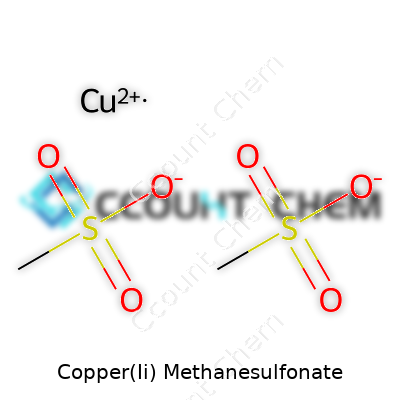
It’s easy to look at a compound’s name and feel overwhelmed, especially with names like copper(II) methanesulfonate. The formula isn’t just random letters and numbers—each piece tells a story. For copper(II) methanesulfonate, the chemical formula is Cu(CH3SO3)2. That Cu stands for copper, the (CH3SO3) part is the methanesulfonate group, and the “2” shows there are two of them for every copper ion.
On my own bench, I’ve handled copper(II) compounds that had industrial jobs, and methanesulfonate stands out. Old habits favor copper sulfate or nitrate, but methanesulfonate offers much higher solubility and produces less toxic waste. Companies searching for cleaner, more efficient ways to plate copper on electronics look to this salt. Those circuit boards in our phones? Many wouldn’t exist in their current form without copper salts like this one to lay down precise metal traces.
It’s hard to ignore pollution. Traditional copper salts can stick around in waste streams and affect water sources. Methanesulfonate, though, creates solutions that are easier to treat. Researchers found that baths built on copper(II) methanesulfonate yield purer metal deposits and simplify the recycling of spent solutions. Compared to sulfate and nitrate versions, this compound’s toxicity profile looks much gentler, offering a safer choice for both workers and nearby communities.
Chemistry classes cover redox reactions, and this salt shows up in those discussions. The copper is in the +2 oxidation state, which drives many reactions. Methanesulfonate acts as a stable anion, clinging tightly to copper and keeping it dissolved. The strong bonds cut down on side reactions, so researchers get more predictable results. The high solubility lets labs and factories run more concentrated solutions, speeding up plating or catalysis. Fewer contaminants build up over time, saving money on maintenance and waste clean-up.
One challenge comes up often: cost. Methanesulfonic acid isn’t as cheap as sulfuric acid, which bumps up production expenses. Small shops sometimes skip these newer copper compounds to keep budgets in check, even though they risk higher waste disposal bills. Regulatory incentives, grants, or partnerships with universities could bridge the gap. Open-source protocols make a difference, too—if labs share tricks and recipes, the costs drop, and startups can get in the game.
On another front, supply chains matter. Factory-scale adoption of copper(II) methanesulfonate depends on access to reliable, high-purity sources. Supporting regional suppliers and investing in better synthesis routes can lower frustration for end users. I’ve watched a lab struggle to find consistent material and resort to DIY synthesis, which slowed down their research. Better support from chemical distributors could ease these bottlenecks.
Even in a field full of exotic organometallics, this compound draws attention for its blend of performance and environmental benefits. Pushing wider adoption hinges on more awareness and investment. Chemistry, at heart, touches our daily lives, and safer copper sources build cleaner technology for all of us. Knowledge of these chemical structures goes beyond trivia—it directs big changes in manufacturing, sustainability, and innovation. Every detail in that formula, Cu(CH3SO3)2, earns its place on the bench and in the world.
Copper(II) Methanesulfonate appears in more labs these days, thanks to its usefulness in electroplating and battery setups. If you’re like me, you’ve probably read the labels and Safety Data Sheets, maybe nodded along, then hoped that your storage habits at work measure up. My first real lesson with chemicals came when I found a corroded shelf and realized that not all bottles are safe to tuck away next to each other. This compound’s no exception.
I’ve seen folks leave metal salts, especially ones like copper compounds, sitting out near the sink, next to acids, sometimes on open benches. Moisture creeps in, and before long you’re staring at a sticky mess or blue-green stains. Just a few grams of this stuff costs more than lunch for the week, so it’s a waste both in terms of money and safety.
Copper(II) Methanesulfonate absorbs water from the air. That means humidity isn’t your friend. In places where summer gets muggy, this can turn manageable powder into caked clumps. When clumping happens, precise measurement gets trickier, and sometimes the chemistry shifts in subtle ways. I saw a team lose an entire afternoon’s prep after discovering their compound had pulled in moisture overnight, throwing off their intended copper concentrations.
Dry, cool storage pays off. Rather than stashing it on the top shelf where heat rises, look for cabinets set away from vents and windows. I always tell new lab members to invest in a desiccator for their shelf – silica gel inside goes a long way and costs next to nothing.
Temperature control can be just as critical. Heat speeds up any slow chemical reactions between air and your compound. If you don’t have a climate-controlled storeroom, aim for a cupboard that doesn’t soak up sunlight. I remember a batch stored near a radiator that turned darker from decomposition products, which meant we had to toss half the stock.
While organizing shelves, I keep oxidizers and reducers far apart. Copper(II) Methanesulfonate doesn’t ignite on its own, but you don’t want a nearby spill with a strong acid or base turning a nuisance into an emergency. Use plastic or glass containers, tightly capped, and avoid metal if you don’t want surprise reactions.
No matter how tight budgets are, resist the urge to reuse food containers. Only containers designed for chemicals with airtight seals should hold this compound. Label every jar and record the date received. Rotating stock so that the oldest gets used first can prevent problems that sneak up with chemical age and slow breakdown.
Hazard labels and safety signs matter, but real safety starts with daily habits. Create a checklist for new deliveries: tight lid, clear label, proper shelf, moisture protection. Share what you learn—the chemist who came before you may never have mentioned that small bottle at the back of the cabinet, but a single slip can endanger the whole workspace.
Copper(II) Methanesulfonate should never become an afterthought, given its value and the risks involved. My own experience says that one overlooked detail—a loose cap or a missed spill—can set you back days or weeks. Make good storage habits your lab’s standard, then pass them along. That’s how we keep the science—and the scientists—safe.
Copper(II) methanesulfonate isn’t a household name, but it finds a place in some industries, mainly electroplating. Anyone who works with chemicals knows: safety information isn’t just a formality on a label. My own time handling various compounds in a university lab taught me that new names often mask tough hazards, so attention to details pays off.
Copper itself is both essential and risky. In small amounts, the body uses copper for healthy blood vessels and nerves. Go above the safe zone and you start seeing problems—headaches, nausea, stomach pain. That copper ion at the center of the compound means it shares some traits with other copper salts. Methanesulfonate, the other half, isn’t particularly toxic by itself, but, joined with copper, the focus stays on the metal ion.
Copper(II) methanesulfonate can irritate your skin and eyes. Breathing dust sometimes brings coughs or a sore throat. Swallowing enough of it definitely doesn’t end well. Copper salts, by nature, show acute toxicity if taken internally—animal studies back that up. Data from the European Chemicals Agency puts the oral LD50 for rats (a standard toxicity test) around 70 mg/kg. That doesn’t mean instant disaster from a single exposure, though. Chronic problems—liver or kidney damage—appear if someone keeps getting exposed over weeks or months.
Long-term studies are slim. You won’t find stacks of published clinical cases from workers using the chemical, but the basics of copper chemistry point to a cautious approach. Of course, we all know regulations can lag behind the science, so personal responsibility matters.
Factories using copper(II) methanesulfonate for plating baths typically manage closed systems. Splashes, leaks, and dust create the most risk, so keeping good ventilation, gloves, and goggles in place makes sense. Some workers I spoke with barely register a concern beyond a “standard rinse” after a shift. That’s fine for those with short contact, but accidents happen fast—skin rashes, eye redness, all reminders that routine dulls caution.
Copper compounds cause trouble in water. Copper(II) ions are toxic to aquatic life; a small release can wipe out fish or invertebrates. That puts pressure on careful waste handling. Storm drains don’t clean up mistakes. I once saw a spilled copper solution turn a small puddle blue—an everyday accident, but problematic if it flows into streams. Local water authorities often set low limits for copper disposal for this reason.
Rules exist for a reason, and so does experience. Basic steps work: protective gloves, goggles, lab coats, enclosed processes, and timely cleanup of spills. Workers need regular training, not just a poster on the wall. Ventilation helps, but personal hygiene—like washing hands before eating—keeps copper out of your bloodstream.
I’ve noticed that open conversations in the workplace about chemical risks make the most difference. If everybody pays attention, from the manager to the new hire, hazardous exposures drop. That, paired with real monitoring—air and water testing—not only prevents illness but also builds trust. Publicly available safety data sheets (SDS) from manufacturers offer solid facts about chemical properties and what to do in emergencies.
Ignoring copper(II) methanesulfonate’s hazards won’t make them disappear. Simple, everyday habits protect lives. Industries that stay alert, invest in training, and share lessons learned raise the standard. That’s something worth building on, for the sake of both people and the environment.
Every chemist comes across moments where they need a copper salt that behaves well in water. Searching for options, Copper(II) methanesulfonate grabs attention because it steps away from classic compounds like copper sulfate or nitrate. The big draw? Solubility. At room temperature, Copper(II) methanesulfonate dissolves freely in water, forming a vividly blue solution. Measured out, labs regularly see solubility greater than 1 mol/L in water, much higher than copper(II) sulfate’s approximate 0.1 mol/L. Copper(II) methanesulfonate’s performance stands out—it skips out on the precipitation or sluggish dissolving that usually slows down copper chemistry.
Handling water-soluble copper compounds gets far easier and more predictable with Copper(II) methanesulfonate. This level of solubility means there’s less fuss mixing stock solutions for applications like electroplating, battery electrolytes, or catalysis research. With copper(II) sulfate or acetate, undissolved particles often clog up filters and lines. With methanesulfonate salts, you see fewer of these headaches, and copper ions end up where you want them—ready for reactions or deposition.
In industry, high solubility boosts the quality and consistency of copper layers during electroplating. If a bath can hold more copper without crystals falling out, operators get smoother, shinier coatings on everything from circuit boards to jewelry. Researchers adjusting electrolyte concentrations see results that track more closely with theory, which tightens up data and saves both time and materials.
There’s more than convenience at play. Methanesulfonate’s non-volatile, inert features mean operators face fewer risks from nasty fumes. Compare that with copper sulfate baths that may toss around sulfuric acid mist, and you get a sense of the safety advantage. Also, fewer solids mean equipment stays cleaner, and disposal of waste gets safer. That can save money in the long run, especially in countries where environmental rules keep tightening.
For folks like me who’ve wrestled with jammed spray nozzles, or spent lunch breaks scrubbing blue residue from beakers, a salt that stays reliably dissolved can tip the scales toward a smoother workday. Lab technicians and process engineers notice those small gains—less time spent fussing over solutions, more reliable test results, and less time wasted on rework.
No chemical is perfect. Even though Copper(II) methanesulfonate dissolves easily, it still introduces copper ions wherever it’s used. Overdosing rivers and soils with copper—no matter the salt—means trouble for fish and microbes. Process managers must think about ways to recapture or recycle the copper load, or at the very least, neutralize waste before dumping it. Closed-loop systems and dedicated copper ion exchange units on site help keep that impact contained.
Any project chasing high copper concentrations in water should recognize the leverage gained from this salt’s solubility. For research, industry, or waste management, folks who handle copper on the regular gain valuable time—and a little peace of mind—using a salt that really makes water work for them.
| Names | |
| Preferred IUPAC name | Copper(II) methanesulfonate |
| Other names |
Copper(II) mesylate Cupric methanesulfonate Methanesulfonic acid copper(II) salt |
| Pronunciation | /ˈkɒpər tuː ˌmɛθ.eɪnˈsʌl.fəˌneɪt/ |
| Identifiers | |
| CAS Number | [128042-82-2] |
| Beilstein Reference | 3449508 |
| ChEBI | CHEBI:91250 |
| ChEMBL | CHEMBL3307428 |
| ChemSpider | 16215677 |
| DrugBank | DB14525 |
| ECHA InfoCard | 03b3ba93-363e-4272-a7c1-511a1dc91aba |
| EC Number | 205-580-9 |
| Gmelin Reference | 23250 |
| KEGG | C02381 |
| MeSH | D017659 |
| PubChem CID | 69504365 |
| RTECS number | GL8575000 |
| UNII | 8OC0Q3P0DX |
| UN number | Not regulated |
| Properties | |
| Chemical formula | Cu(CH3SO3)2 |
| Molar mass | 241.7 g/mol |
| Appearance | Blue powder |
| Odor | Odorless |
| Density | 1.957 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.9 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 6.2 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Refractive index (nD) | 1.485 |
| Dipole moment | 4.559 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 206.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -769.9 kJ/mol |
| Pharmacology | |
| ATC code | V03AY03 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H334, H335, H351, H373, H410 |
| Precautionary statements | P234, P264, P270, P273, P280, P302+P352, P305+P351+P338, P310, P321, P332+P313, P362+P364, P391, P501 |
| LD50 (median dose) | LD50 (median dose): Oral, rat: >2000 mg/kg |
| PEL (Permissible) | PEL (Permissible exposure limit) for Copper(II) Methanesulfonate: 1 mg/m3 (as Cu dust and mists) |
| REL (Recommended) | 0.01 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Copper(II) sulfate Copper(II) nitrate Copper(II) chloride Copper(II) acetate Copper(I) methanesulfonate Methanesulfonic acid |