Curiosity in the field of organofluorine chemistry drove research toward compounds packing a punch in stability and reactivity. Calcium Bis(Trifluoromethanesulfonate), sometimes better known as calcium triflate or Ca(OTf)2, emerged out of a search for versatile, robust salts during the late 20th century. Early references in global chemical literature show how innovators noticed that the triflate group delivered both extraordinary chemical stability and a knack for dissolving in polar environments. The growth of green chemistry and demand for high-purity electrolytes fueled the research pipelines. Over time, this material graduated from being a scientific curiosity to an essential ingredient in battery research and novel catalysis.
At a glance, calcium triflate looks unassuming—a fine, white, odorless powder that packs a wallop in performance. The main draw comes from its unique structure, which links calcium ions with two hefty, electron-rich triflate anions. Industries dealing in electrolyte development, chemical synthesis, and analytical chemistry rely on this compound for its stability, high solubility in organic solvents, and low nucleophilicity. Reputable chemical suppliers list a range of purities, often exceeding 98% for research-graded stock. Packaging varies by application, but moisture-proof containers remain the standard since even trace humidity can cause clumping and reduce reactivity.
Solid at room temperature, white and powdery, this compound melts well above 300°C, which allows it to handle laboratory heat cycles with ease. Cali-um Triflate’s solubility profile opens plenty of doors: it dissolves strongly in acetonitrile, dimethylformamide (DMF), and many alcohols. In water, it holds a moderate solubility, making it popular in aqueous and mixed-solvent work. The molecular weight runs about 446.28 g/mol. Unlike many simple salts, this compound resists breakdown in both acidic and basic environments—so it won’t fall apart during synthesis or storage. The triflate anion adds both chemical and oxidative toughness, making this salt a dropped penny that won’t tarnish.
Manufacturers back up their promises with data—certificate of analysis sheets confirm purity, water content, and trace metals. Responsible vendors ship the compound in sealed, nitrogen-filled bottles, with detailed GHS-compliant labeling. Label information should always include the systematic name, lot number, molecular formula (Ca(CF3SO3)2), and hazard statements. Routine ICP-OES or ion chromatography measures contaminants, and NMR confirms structure. Look for labels calling out low moisture content and absence of magnetic or ferrous impurities, as many uses in batteries or catalysis demand these assurances.
Synthesis starts with calcium carbonate, calcium hydroxide, or calcium chloride, which reacts with an excess of trifluoromethanesulfonic acid. For instance, adding the acid to a slurry of calcium carbonate results in CO2 bubbles escaping, while calcium triflate precipitates. Washing and vacuum drying the product prepares it for commercial use. Some processes call for purification by recrystallization from acetonitrile or methanol, stripping away magnesium, sodium, or other metallic by-products. Laboratory-scale production often scales effortlessly since the reagents are commercially available and the method avoids excessive heat or hazardous solvents.
In organic synthesis, calcium triflate acts both as a Lewis acid and a mild, stable electrolyte. Organic chemists depend on its ability to activate carbonyl compounds, facilitating reactions like aldol and Michael additions. The compound rarely introduces unwanted side groups since the triflate ions resist nucleophilic attack, making reactions more predictable. Some advanced research teams modify calcium triflate complexes for tailored reactivity in catalysis or materials science, often replacing half the triflate content with other anions. One popular research angle explores doping solid-state electrolytes with this salt, chasing safer, more stable battery chemistries.
Across catalogs and journal articles, Calcium Bis(Trifluoromethanesulfonate) shows up under a parade of aliases. You’ll see it written as calcium triflate, calcium trifluoromethanesulphonate, and names abbreviated as Ca(OTf)2. Some suppliers recycle branding like “HiPure Calcium Triflate” or “AnhydroTriflate Ca,” but chemically, they all point to the same salt. Regulatory databases link it to CAS Number 42199-29-9. Knowing the precise name helps locate technical data and ensures quality, especially for regulatory filings or safety documentation.
Work with calcium triflate stays safe when using routine personal protective equipment: gloves, goggles, and a dust mask. The compound won’t burn skin, but it can irritate mucous membranes, so splash protection matters. Dust generation poses a respiratory risk, so use inside a hood. Spills usually sweep up cleanly, with waste containers marked for fluorinated organics. The salt won’t combust or deliver an acute toxic punch, but its chronic effects lack long-term study. Always check safety data sheets for latest legal and handling updates, especially when moving into gram-scale or pilot plant work. Storage in airtight, moisture-free containers prevents caking and guarantees shelf life.
Calcium triflate works as the MVP in organic synthesis—pharmaceutical labs deploy it as a Lewis acid to run cleaner reactions with greater selectivity. Research in electrochemistry leans hard on this compound, since the salt powers calcium-ion and magnesium-ion battery cells due to its high ionic conductivity and oxidative toughness. Scientists in catalysis rely on its low nucleophilicity to steer reactions without unwanted side reactions. Its performance stands out in polymerization, peptide synthesis, and even water treatment trials. There’s fresh excitement as greener energy storage becomes a global goal, with calcium cells using this very salt as a cornerstone electrolyte, outperforming outdated options.
The pace of research on calcium triflate rivals anything in modern chemistry. Electrochemists build new battery cells that replace lithium with calcium, chasing lower cost and safer grids. Material scientists probe its effects in novel supercapacitor blends. Drug discovery teams use the salt to drive carbon-carbon coupling, taking advantage of both its reliability and minimalist waste stream. Industry partnerships spur joint projects and funding, with recent government grants backing greener, less hazardous electrolyte chemistries. Analytical chemists write up protocol improvements in trace metal detection, leaning on the salt’s clean background signals. Patents linked to solid-state electrolytes and smart polymer systems name calcium triflate as a backbone material, confirming its presence at the innovation table.
Toxicology studies still lag behind the industrial adoption rate. What’s known: acute oral and skin toxicity remains low in rodents, with no obvious mutagenic or carcinogenic signals in standard assays. Chronic exposure to triflate anions has drawn closer scrutiny, as fluorine-heavy molecules can sometimes slip into biological pathways where they don’t belong. Modern regulatory filings report that dust exposure and water runoff deserve containment—bioaccumulation in aquatic systems poses a possible risk if production outpaces environmental controls. Continued funding for independent toxicity studies feels critical, especially as wider adoption in consumer tech draws public health interest.
Next-generation batteries and clean-energy tech projects depend heavily on robust, reliable conducting salts. Calcium triflate’s combination of stability, conductivity, and ease of production puts it on the fast track for major adoption in grid-scale energy storage. Ongoing projects aim to further cut trace metal contamination and lower synthesis temperature, making it even more sustainable. Some research teams experiment with hybrid salts, blending calcium triflate with organic and inorganic partners to chase tailor-fit chemical properties. As energy and pharmaceutical sectors grow more urgent about sustainability, demand for high-purity, low-impact electrolytes will put even more focus on this compound. The way researchers and industry respond—by investing in safer handling, clearer toxicity data, and greener processes—will shape just how big a role calcium triflate plays across multiple industries into the next decade.
Working in the lab, certain chemicals develop a reputation. Calcium bis(trifluoromethanesulfonate) doesn’t grab headlines, but anyone doing organic synthesis or battery research recognizes the name. Its appeal isn’t flashy. Chemists reach for it because it solves problems traditional salts can’t touch. The secret is in the “triflate” part. Triflate anions show rare stability and resist messing up sensitive reactions. This matters more than you think: a catalyst tainted by unwanted byproducts sends an entire batch down the drain—wasted time, money, and materials.
Back in grad school, I learned that certain reactions stall without a little help. Running a Friedel-Crafts acylation? The right salt unlocks tough bonds, allowing molecules to snap together. That’s where calcium bis(trifluoromethanesulfonate) enters. Using this compound as a Lewis acid, organic chemists boost yield and skip over frustrating “dead ends” that weaker, less specialized salts create. Even in classic synthesis textbooks, calcium triflate earned a couple of shoutouts. That’s no small feat, considering how rarely textbooks mention newer reagents.
People talk a lot about lithium-ion these days, but folks in advanced materials labs keep exploring alternatives. Calcium batteries attract attention because calcium is cheap and more abundant than lithium. Keeping the electrolyte stable is the tricky part. Calcium bis(trifluoromethanesulfonate) stands out for high ionic conductivity and stability at different voltages. Research papers out of Japan, Germany, and the U.S. cite calcium triflate when looking for that elusive mix of safety, performance, and long life. One group tested this compound and found it let calcium ions travel easily between the battery’s anode and cathode, reducing degradation and improving performance. This isn’t just theory—it’s one of the practical steps researchers take to move beyond the lithium squeeze.
Newer chemical processes tangle with environmental rules and safety expectations. Calcium triflate comes up in discussions because it doesn’t carry the high toxicity or persistence of some older salts. In pharmaceutical research, for example, the cleaner the chemistry, the easier regulatory approval becomes. A safer salt reduces downstream problems during scale-up and cleanup, so companies searching for greener methods pay attention. I’ve heard researchers at process chemistry conferences mention swapping out old standbys for calcium triflate just to keep their waste streams less hazardous and easier to process.
Cost and limited large-scale data slow down wider adoption. Calcium bis(trifluoromethanesulfonate) can run expensive, especially for big plants outside the laboratory. Sourcing high-purity material gets tricky, and nobody wants surprise contaminants in their product batches. That said, manufacturers dedicated to reproducible, tight-spec chemicals have started scaling up. Lower prices could follow, if the demand keeps rising from battery and pharma giants.
As someone who’s watched chemists trade stories at the bench, the jury isn’t out on calcium triflate’s ultimate impact. Still, it’s more than a niche curiosity. Whether you’re a grad student trying to finish a stubborn synthesis or an engineer hunting for the next battery breakthrough, this salt holds promise.
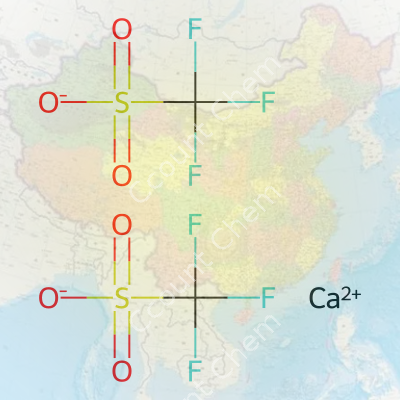
Calcium bis(trifluoromethanesulfonate) goes by a mouthful of a name in the lab, but behind it hides a pretty tidy formula: Ca(CF3SO3)2. For anyone tinkering with battery electrolytes, organic synthesis, or electroplating, this compound tends to show up more often than expected. Each molecule packs one calcium ion and two trifluoromethanesulfonate anions, which is why the formula carries those twos after the parentheses. This formula matters. In chemistry, even the smallest mistake can send a perfectly good experiment sideways.
Formulas work like language. I remember in university, weighing out a so-called calcium salt for a project, but trusting a Google search that dropped a “2” from somewhere. I ended up with something that wouldn’t dissolve, much less help me build a working battery. That embarrassment lingers, and it highlights the value of clean notation. If scavenging for calcium salts, Ca(CF3SO3)2 brings two anions for every calcium atom, keeping charges nicely balanced. Miss that—even slightly—and the results turn confusing fast.
This salt features in non-aqueous electrolytes where waterless conditions allow for stable charge transfer. Scientists like it because triflate salts, like this one, dissolve well in organic solvents and stay chemically stable even when voltages rise. Research shows that Ca(CF3SO3)2 supports cation transport with fewer side reactions than earlier calcium salts. In industrial electrochemistry, this reliability is gold. If building a prototype battery or running a reaction at scale, the exact makeup of the salt will decide whether the process hums along or grinds to a halt.
Mislabeling formulas leads to wasted time and wasted chemicals. Plenty of chemists have ordered a “calcium triflate” from a supplier, trusting they’d get the right anion, only to spot surprises when things don’t react as expected. Trusting in exact naming and formula writing stops those small mistakes from snowballing.
There’s also confusion because names in catalogs can leave out details. “Calcium triflate” might sound broad enough, but without the full formula, it’s possible to end up grabbing a different salt that acts differently in the beaker. Standardizing chemical inventory systems and pushing for full formula display helps people double-check before pouring anything in. Big journals and reliable suppliers usually do it right, but I’ve seen smaller vendors slip.
Relying on detailed product labels, double-checking technical datasheets, and using reputable sources make a difference. Labs can run in-house checks—like routine NMR or IR spectra—if the stakes feel high. Many chemistry departments now teach incoming students to question labels and look for precise IUPAC names alongside formulas. That sort of vigilance plays a bigger role each year, especially as synthetic routes or battery recipes grow more demanding.
The lesson that sticks with me: chemistry starts with tiny details, and those details begin with the right formula. Ca(CF3SO3)2 isn’t just a run of odd symbols; it’s the whole starting point for clean reactions, clean data, and fewer headaches down the road.
Most people don’t come across calcium bis(trifluoromethanesulfonate) during their daily routine. For chemists and science teachers, though, this compound pops up often enough to matter. The question keeps landing in research forums and classroom discussions—does it dissolve in water, and does that matter for anyone outside a university lab?
You mix some calcium bis(trifluoromethanesulfonate) with water, and yes, it dissolves. Unlike old-school salts like calcium chloride, which disappear into a beaker without a fuss, this compound stays visible a little longer, but it gets there in the end. Its trifluoromethanesulfonate parts—so-called triflates—draw aqua molecules and break apart, letting the calcium ions move freely in the solution.
You get a solution that’s clear if you start with the right grade. No odd fizz, no murky mess. If a student asks why it matters, I point to its structure. The triflate group, loaded with electronegative fluorines, yanks electrons and keeps things stable. This boosts solubility in polar solvents like water. Other calcium salts hog the limelight in daily life, but chemists appreciate how this one offers both solubility and low reactivity. It gives more control in experiments without unpredictable side reactions.
On paper, the question looks dry, but it calls for practical answers. Pharmaceutical chemists sometimes need a source of calcium that peacefully shares space with complex molecules—no nosy anions making a mess. Others in battery research look at calcium bis(trifluoromethanesulfonate) as a possible salt in next-generation batteries, where water solubility becomes vital for safe, efficient processing. Materials scientists get another safe calcium anchor for polymerization reactions.
People in these fields look for reliable data because their experiments depend on it. You find journal references showing solubility tests, and everyone involved gets the same message: dissolve it in water, and it plays along. If you ever tried dissolving calcium sulfate or phosphate, you see gritty leftovers and a cloudy mess. With calcium bis(trifluoromethanesulfonate), none of that hassle.
The story does not stop just with solubility. Price is a big hurdle. This compound doesn’t come cheap, especially in the purities needed for advanced research. If you work on a tight research budget, you think twice about picking it over cheaper alternatives. Environmental questions creep in, too. With all those fluorines, people worry about downstream waste lingering in waterways. That concern isn’t unique to this compound—lots of fluorinated chemicals force a closer look at disposal rules.
What I see from my own years in lab education: Students and early researchers rely on clear, direct sources. Sometimes the information hides behind paywalls or gets buried in product catalogs that do not clearly mention how easy or reliable dissolution is. Building a better database or at least publishing more open-access data helps everyone, and it boosts confidence when the compound lands in the hands of non-specialists.
We need more plain-language guides for chemicals like this, aimed at teaching labs as well as research teams. Safer alternatives—perhaps new calcium salts—should get funding and attention, too. And anyone who orders, uses, or disposes of fluorinated compounds ought to have clear, up-to-date safety protocols. A compound’s solubility opens doors, but keeping those doors safe for everyone should remain front and center.
Lab work often feels like an endless series of decisions: which solvent to use, how to handle temperature changes, what kind of labels to slap on a container. If you use calcium bis(trifluoromethanesulfonate) — a mouthful that’s sometimes called calcium triflate — storing it the right way isn’t just a textbook recommendation. It’s a clear step for personal safety, experiment integrity, and budget control.
Calcium triflate draws water from the air like a magnet. If left out or sealed in containers with too much headspace, the salt soon turns clumpy or even dissolves itself. I saw this the first time I worked with it, thinking a regular plastic container would do the trick. It ruined the material and spoiled a week’s worth of follow-up tests. The salt pulls in humidity even in low-moisture labs.
So, best practice lines up with facts: keep calcium triflate in tightly capped glass bottles or high-quality plastic vials. Glass works well against vapor escape. Desiccators — those dry storage boxes with silica gel or other drying agents — give a big advantage. Even when labs get humid (every summer in a poorly ventilated building), a desiccator keeps things dry and stable. These small steps prevent clumping and loss of reactivity, so batches last as long as promised on paperwork.
Heat speeds up chemical changes across the board, and light can do its own damage, especially after weeks on the shelf. I remember a colleague’s sample, forgotten on a sunny bench, collecting a strange crust after just two days of sunlight. Chalk this up as a reason to store the salt in cool, shaded cabinets or drawers, nowhere near oven exhausts or busy windows.
Room temperature works for most labs. Refrigerators get used when humidity battles the best caps. I’ve found it useful to label containers with open dates and last-use notes. Routine checks for leaks or crusts save time, money, and — most of all — headaches during crunch times.
Sloppy labeling creates mix-ups that throw off results and cost cash. Cross-contamination gets real if the same scoop touches multiple bottles. Always grab a dry, clean spatula or scoop and make sure hands stay gloved. Write dates and lot numbers on each bottle. If you pull small portions out regularly, split them up in advance to avoid condensation ruining the bulk supply.
Spills of calcium triflate don’t spark fires, but they can make a mess. Clean counters fast with water and dispose of residues through standard chemical waste streams. Don’t let the salt work into porous benchtops; it’s tough to clean once inside tiny cracks.
Reliable chemistry grows from the little habits: tight lids, dry boxes, cool shelves, and clear labels. Talking to colleagues and sharing updates on storage methods helps others avoid repeats of old mistakes. It’s far better to spend a few extra minutes on storage than rush to salvage ruined chemicals later. Calcium triflate isn’t cheap, so every gram kept active translates to better data and less wasted money — a simple, proven win for anyone working at the bench.
Calcium Bis(Trifluoromethanesulfonate) shows up in research labs for its role as a catalyst and electrolyte, especially in organic synthesis and battery technology. It’s attractive for its unique properties, but the compound brings real safety concerns to any workspace. Working around materials like this in labs over the years, it becomes clear that real safety isn’t about memorizing guidelines; it’s about weaving safety into each step of the routine. One overlooked slip can cause big trouble, both for people and equipment.
Lab coats, safety glasses, and chemical-resistant gloves should always be on. Regular nitrile gloves hold up well, and a face shield matters if splashes might happen. Skin contact leaves a risk of chemical burns, and accidental splashes near the eyes can do permanent damage. I’ve seen simple glasses stop what could have been a hospital trip. Respirators don’t usually come out unless dust or fine powder threatens the air, but keeping one close can’t hurt if large amounts get disturbed.
Even without a strong smell, chemical dust drifts up while handling the powder. Fume hoods aren’t just a box in the corner. Any weighing, mixing, or transferring of this compound happens under a good fume hood. Not every lab has perfect airflow, and it only takes one small spill for irritation or breathing problems to hit. At one crowded facility, a single scoop onto the benchtop led to coughing fits and a lot of grumbling about cleanups. It just reinforces how essential working under extraction keeps everyone safe.
A dry, cool, well-ventilated cabinet holds calcium bis(trifluoromethanesulfonate) safely. Moisture from humid rooms can mess with its stability, so tight containers really matter. In the lab, someone once thought a snap-top jar counted as “close enough,” and days later we found a clumped mess. Labels with clear hazard warnings prevent mix-ups and quick decisions during emergencies. Cleaning up spills calls for a dedicated spill kit—no grabbing the nearest rag. Small quantities lift readily with a damp absorbent, sliding right into a sealed plastic bag for hazardous waste pickup.
Too many accidents come from rushing or cutting corners. Training every team member (even the experienced ones) helps keep up strong habits. This chemical rarely causes drama in headlines, but the potential for skin and respiratory harm makes training worth every minute. Once someone skipped basic safety checks while prepping for a late-night run, simply because it “seemed fine last time”—regretted as soon as powder hit an open soda can nearby and fizzed up. Avoiding food or drinks anywhere near chemical handling space isn’t just a rule; it saves stomachaches and ER visits.
Disposing of this compound safely follows strict rules. Dumping waste down the drain never cuts it. Chemical leftovers and contaminated gloves go into marked hazardous waste bins. Regular pickups by trained contractors take care of the rest, keeping the lab and environment protected. Nobody wants a regulatory visit finding a hidden jar under a bench.
People working with chemicals need more than checklists—they need awareness. Following safety rules for calcium bis(trifluoromethanesulfonate) isn’t busywork; it means respecting both your health and the people around you. Learning from every near-miss or small mistake builds better habits and keeps the lab running safely tomorrow.
| Names | |
| Preferred IUPAC name | calcium bis(trifluoromethanesulfonate) |
| Other names |
Calcium trifluoromethanesulfonate Calcium triflate Calcium trifluoromethanesulphonate Calcium(II) trifluoromethanesulfonate |
| Pronunciation | /ˈkæl.si.əm bɪsˌtrɪ.fluː.roʊˌmiː.θeɪnˈsʌl.fə.neɪt/ |
| Identifiers | |
| CAS Number | 42199-05-1 |
| Beilstein Reference | **4-02-00-01838** |
| ChEBI | CHEBI:91249 |
| ChEMBL | CHEMBL1924720 |
| ChemSpider | 21843880 |
| DrugBank | DB11240 |
| ECHA InfoCard | 01d2a38a-10e6-4b5a-8d7d-f3193aff9b41 |
| EC Number | 208-766-5 |
| Gmelin Reference | 608934 |
| KEGG | C19245 |
| MeSH | D018364 |
| PubChem CID | 25147576 |
| RTECS number | WN6090000 |
| UNII | 31J16QG6E6 |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID20897786 |
| Properties | |
| Chemical formula | Ca(CF3SO3)2 |
| Molar mass | 398.24 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.96 g/cm³ |
| Solubility in water | soluble |
| log P | -2.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | -2.5 |
| Magnetic susceptibility (χ) | -70.0e-6 cm³/mol |
| Refractive index (nD) | 1.390 |
| Dipole moment | 1.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 453.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2024 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Explosive limits: Non-explosive |
| LD50 (median dose) | LD50 (oral, rat): >5000 mg/kg |
| NIOSH | TT9627000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Aluminium trifluoromethanesulfonate Magnesium trifluoromethanesulfonate Lithium trifluoromethanesulfonate Potassium trifluoromethanesulfonate Sodium trifluoromethanesulfonate |