Chemists first set their sights on organoiodonium compounds about a century ago, but their true potential only hit the spotlight in the electronics boom of the late twentieth century. As microcircuits got smaller and photolithography processes pushed for finer features, researchers realized traditional photoinitiators couldn't keep up with the demand for higher resolution and sensitivity. Progress rolled along with the work of Japanese and American teams in the 1980s, who optimized bulky diaryliodonium salts for industrial and scientific labs. The introduction of nonafluorobutanesulfonate as a counterion marked a jump in solubility and thermal stability. Over the years, innovations expanded use well beyond chip fabrication, growing into cationic polymerization, advanced coatings, and materials science.
Bis(4-tert-butylphenyl)iodonium nonafluorobutane-1-sulfonate falls into the category of onium salts. Anyone familiar with photopolymerization or lithography likely ran across this reagent. The large, non-coordinating nonafluorobutanesulfonate counterion helps drive high solubility in common organic solvents—quite the advantage in modern resin and coating formulations. The compound works as a photoacid generator, meaning it releases acid when hit by UV or electron-beam energy. This function unlocks a cascade of downstream chemical changes on the molecular level, required in devices demanding accurate structural definition.
You’ll spot bis(4-tert-butylphenyl)iodonium nonafluorobutane-1-sulfonate as a white to off-white powder—dense and somewhat waxy to the touch. It doesn’t have a strong odor, a welcome perk for anyone handling kilos per year. With a melting point that can hold north of 160°C and notable stability even in slightly damp conditions, it stores well. The compound offers robust solubility in solvents like acetone, acetonitrile, and DMF, but less so in pure water. Its molecular structure brings together a bulky, electron-rich aryl group and a heavily fluorinated sulfonate, which bolsters both thermal durability and assists with uniform dispersion in polymers.
Quality control rarely slips with reliable suppliers. Specs often call for a purity above 98%, which keeps unwanted side reactions at bay. Major packaging labels point out the product’s moisture level (ideally less than 0.2% water content), recommended storage temperatures (below 30°C usually does the trick), lot number, and chemical batch traceability. Detailed certificate analysis accompanies commercial batches. Many packaging drums use UV-blocking materials to safeguard against accidental light exposure—even brief stray sunlight can spoil an entire shipment. Chemical labeling aligns with UN transport and GHS standards, clearly marking irritant and environmental safety statements.
Making this iodonium salt starts with a reaction between 4-tert-butyl iodobenzene and an oxidant, with silver nonafluorobutanesulfonate often leading as the counterion source. The oxidant (such as m-chloroperoxybenzoic acid or Selectfluor) provides the kick needed for iodonium center formation. Careful reaction temperature control avoids side products. Workup includes filtration to strip away silver halides, extraction using safe-to-handle organic solvents, and finally solvent evaporation under vacuum. The last steps lean heavily on recrystallization, which clears away impurities and moisture that could sabotage consistent photoinitiator performance.
The iodonium group cracks open a range of synthetic doors. It generates strong acids upon UV irradiation, which rapidly trigger ring-opening polymerizations (think of epoxies or vinyl ethers hardening in seconds). In organic synthesis, iodonium salts have carved out a niche as mild yet effective arylating reagents. With some creative tweaks, researchers have built tailor-made derivatives for fresher challenges—by swapping the tert-butyl group or by adjusting sulfonate chain length, they redefine the product’s solubility profile and response to light. Several labs have reported success using metal-catalyzed cross-coupling reactions, coaxing these iodonium compounds into building up more complex aromatic systems. Many industries care about the byproducts: releases only volatile and non-toxic fragments, with low residue risk.
Nothing breeds confusion quite like overlapping nomenclature. Besides the full IUPAC name, most suppliers use shorter names like “bis(4-t-butylphenyl)iodonium NfO”, “BTBPI-Nonaflate”, or “BTBPI-NfO”. Synonyms in non-English catalogs sometimes appear, though they generally keep close to the English-rooted forms for global trade. In research papers, many authors abbreviate to “BTBPI NfO” or “BTBPIN”, but CAS number tracking usually clears up ambiguities. Any new regulation or patent tends to list at least two common synonyms for quick identification.
Real-world applications demand respect for both reactivity and possible hazards. Even if the acute toxicity runs low, material safety data sheets call for gloves and goggles at the lab bench. The product irritates eyes and skin—direct contact can’t just be brushed off. Spills need rapid cleanup with spill pads rather than sweeping dust back into the air. In heated reactions, the compound may break down, releasing small quantities of corrosive gases. Storage means keeping the material in dry, opaque containers, away from acids, bases, and ignition sources. Chemical waste must never reach municipal water; approved hazardous waste handlers usually manage disposal under regional laws.
Anyone working in the microelectronics field or studying polymer chemistry likely encountered iodonium salts. The rise of photoresists in microchip manufacturing came alongside demand for high-efficiency, low-contaminant photoacid generators—and BTBPI NfO fits that niche with its sharp acid release and clean, residue-free breakdown. It pops up in cationic UV-curing resins, where it cuts curing times drastically and sharpens pattern fidelity. Some companies have piloted its use in high-gloss, tough-as-nails coatings for automotive or aerospace components. More recently, research highlights its promise in 3D printing resins and even dental composites, seeking better control over depth of cure with minimum thermal stress.
R&D teams keep chasing better polymerization outcomes, higher sensitivity to shorter UV wavelengths, and improved environmental performance. University and industry labs keep tweaking both the iodonium and the sulfonate pieces, searching for formulas that suit specific end uses. Analytical teams carry out photochemical lifetime studies, mapping the quantum yield of acid production under different light sources—from soft UV LEDs to hard EUV exposure units. Collaborations with computational chemists guide which molecular changes might bump up performance while reducing cost. In recent international conferences, presenters explored BTBPI NfO’s compatibility with renewable biopolymers, opening doors to more sustainable photoinitiator platforms.
Studies so far put BTBPI NfO well below classic industrial hazards, but regulators look closely at any persistent, fluorinated product. In small animal testing, the compound triggers only minor skin and eye reactions at high exposure, though chronic environmental fate remains a question—especially with the fluorinated anion’s potential to accumulate. Environmental monitoring groups keep a sharp eye on discharge levels from manufacturing sites. Newer formulations experiment with shorter-fluorinated or hybrid counterions, aiming to cut environmental persistence while keeping the benefits of non-coordinating anion chemistry. Public databases show that, with proper handling and disposal, risk to workers and the public rests on solidly managed ground.
Tomorrow’s breakthroughs depend strongly on radical innovation in electronics, renewable materials, and green chemistry. As fabrication nodes shrink and demand for high-fidelity patterning expands, next-generation photoacid generators will continue to push the envelope on sensitivity, shelf life, and environmental safety. The core design behind BTBPI NfO seems ready for adaptation; tweaks in both organoiodonium and anion design could help transition this compound into biodegradable or biocompatible platforms. Teams across Asia, Europe, and North America continue to file patents for new variants, each with claims of faster curing, wider light window, or minimal ecological footprint. From a chemist’s perspective, the ideal future holds a suite of options, allowing engineers to match the perfect photoinitiator to any scenario—without sacrificing safety or cost. The story of BTBPI NfO tracks the broader sweep of functional chemistry: innovation driven by real-world needs, always pressing toward better performance and higher responsibility.
Walking into a research lab or a manufacturing floor, you’ll see racks of chemicals with names that only a handful of folks memorize. Bis(4-Tert-Butylphenyl)Iodonium Nonafluorobutane-1-Sulfonate stands out, not for being catchy, but for the value it brings to modern industries. Not everybody has heard of it, but plenty of engineers rely on it more often than they realize.
Spend enough time in the world of electronics or photolithography, and you come across the term “photoacid generator.” That’s the job Bis(4-Tert-Butylphenyl)Iodonium Nonafluorobutane-1-Sulfonate takes up. Under ultraviolet light, this compound releases acid. That might sound like a neat party trick, but it drives a lot of progress in chip manufacturing. UV light triggers a chemical change, letting technicians craft intricate patterns onto silicon wafers, which form the heart of microchips.
You see these chips everywhere, from smartphones to medical devices. The compound’s role might be small, but the stakes run high. If chip patterns lose their precision, product yields drop and costs climb. In an industry where a tiny error can scrap millions of dollars’ worth of work, every process step counts.
Head over to advanced printing workshops, and technicians deal with the same category of chemicals. Photoacid generators help in creating high-resolution imagery on special films. Chemists mix Bis(4-Tert-Butylphenyl)Iodonium Nonafluorobutane-1-Sulfonate with light-sensitive materials to ensure crisp edges and clear details. That’s a must for printed circuit boards and specialty packaging.
I’ve seen production lines stall because some printer ink smeared just a fraction under the microscope. Customers notice, and complaints pile up. Reliable, selective chemical reactions help sidestep those headaches.
The chemical earns a spot in sensitive processes because it’s predictable. Its nonafluorobutane sulfonate part makes the whole molecule dissolve well in certain solvents, which means a more uniform distribution across surfaces. The tert-butylphenyl side groups also help cut down on unwanted byproducts. The upshot: fewer impurities, better performance.
A research colleague told me that, after switching to this specific iodonium salt, defect rates in his team’s micro-patterns dropped by nearly 30%. That’s real progress you can’t just chalk up to luck.
Specialty chemicals usually demand careful handling. Photoacid generators don’t belong anywhere near food or open water because they break down into strong acids. Regulations push companies to find safer, more sustainable production paths. Environmental groups keep a close watch on disposal and emissions. Some countries now require end-users to report how they minimize residual chemical waste after production.
In my experience, training and strict protocols make all the difference. Proper safety gear, real-time monitoring, and strong relationships with recycling partners do more than satisfy paperwork—they keep accidents out of headlines.
People keep searching for greener alternatives, but for now, Bis(4-Tert-Butylphenyl)Iodonium Nonafluorobutane-1-Sulfonate isn’t leaving the scene. Manufacturers invest in recycling and capturing leftover acid releases. Some facilities have moved toward closed-loop systems, letting them reduce spills and keep processes clean.
The way we rely on specialty chemicals keeps shifting, but the need for safe, predictable, high-performing photoacid generators stays locked in. Anyone who ignores advancements in handling or disposal falls behind. Chemical companies who build trust around those improvements earn respect, not just contracts.
Many people overlook storage once a product lands at home or in the back room of a shop. In reality, what happens after purchase can shape safety, shelf life, and even health outcomes. I’ve seen this in both my family’s grocery store and at home. Product storage plays a much bigger role than most expect.
If you toss medicine into a steamy bathroom cabinet, you can ruin tablets quicker than you think. Vitamins, food, even cleaning supplies face similar risks. Storage isn’t just about keeping things out of sight — it protects what’s inside the package. Failing to follow basic instructions can spoil days, weeks, or even months of shelf life, with both money and well-being lost along the way.
Staples like flour, rice, and oatmeal all expect a cool, dry spot. Warmth or rising humidity brings in molds and bugs. I learned this lesson after losing a whole sack of rice during a humid summer. Fruit doesn’t last long lined up by a sunny kitchen window, either. Apples and potatoes stay firmer in a dark pantry. For perishable food, consistency wins the race — refrigerators hold temperature better if not crammed full or left open. Pay attention to “use by” dates, but also to signs of spoilage. Trust your eyes and nose.
Fresh meat calls for quick refrigeration or freezing. Milk keeps longest if it’s stored in the main body of the fridge, not the door. Dairy and eggs need a stable, cool temperature. Freezing is a good option for items you can’t finish in time. I’ve seen many folks forget that thawing on the kitchen counter brings bacteria out of hiding, so always thaw in the fridge.
Medication presents a different set of risks. A warm glove compartment or sunny bathroom shelf will break down pills faster than you think. Most medicine calls for a dry, stable room temperature — usually between 15 and 25 degrees Celsius — with some exceptions for products requiring cold storage. If you’re unsure, ask your pharmacist or consult the packaging directly. Never mix bottles or pull tablets out of blister packs in advance. Originals protect better than homemade pill containers.
Cleaning solutions work best if they stay sealed, upright, and away from heat. Never store flammables — like bleach or alcohol-based sprays — near a heater, oven, or direct sun. Safe storage keeps poison away from kids and pets, too. I keep these items on a high shelf, in a clearly marked cabinet with a secure latch.
Labels deliver important clues. Keep products in their original containers with safety instructions visible. Mixing or pouring into unmarked bottles has led to some close calls and emergency room visits.
Many problems boil down to poor habits. Make a checklist of storage spots and review it every few months. Invest in airtight jars for pantry goods. Use hygrometers and thermometers in uncertain areas like garages or sheds. Simple shelving, clear containers, and proper labeling all support safer storage. Take time to read directions on packaging or ask experts. It’s a small investment that saves money, hassle, and sometimes even lives.
Lab work rarely tolerates compromise. When working with Bis(4-Tert-Butylphenyl)Iodonium Nonafluorobutane-1-Sulfonate—a mouthful, but an important photoacid generator—the purity question isn’t just academic. As someone who’s had to troubleshoot mysterious results for hours, I can tell you that contamination, even on a tiny scale, runs roughshod over reliable outcomes.
With most commercial suppliers, you’re looking at reported purities in the neighborhood of 98% or higher, usually measured by HPLC and NMR. This doesn’t mean only one thing: a percentage on a label sums up a story of what’s present in that bottle. If you’ve ever watched a chromatogram twist and spike from lurking byproducts or solvents, you learn to respect these numbers. A peak where there shouldn’t be one spells issues for precision photolithography or microelectronic fabrication. The industry standards only go so far—solvents, water, inorganic halides, or organics left over from synthesis keep sneaking in.
Small percentages of impurity might seem negligible in theory. In practice, they often trigger headaches: reduced sensitivity, unexpected side reactions, or stubborn background noise during patterning steps for electronics. Imagine designing an ultra-clean circuit, and somewhere in the process, an impurity shifts the energy absorption, cutting the process latitude. Extreme cases pile on costs for recycling wafers or re-making batches.
After seeing the swings in purity from batch to batch, and even within suppliers, my takeaway is clear. Lab techs start valuing consistency as much as high numbers on a COA. Reputable producers share extensive analytical data on the batch, not just the compound “in theory.” Anyone sourcing this iodonium salt for large-scale or crucial tests gets wise about checking lot-specific details, or requesting extra verification runs—sometimes even running their own tests rather than taking purity claims at face value.
Reducing risk starts by locking in strong supply relationships and prioritizing transparency. It helps to encourage suppliers to invest in better purification technologies and routine upgrades to their quality control processes. Lab teams collecting unused samples for future check-ins often catch issues before big losses kick in. Investing in a benchtop NMR or setting up a partnership with a trusted analytic lab pays off, especially when expanding to pilot or production runs.
Increased demand for ever-smaller, ever-more-precise devices keeps raising the bar. What felt “pure enough” a few years ago doesn’t cut it anymore, and the field doesn’t forgive sloppiness. Good documentation, vigilance, and a little stubbornness often save the day—if only more of us had learned those lessons earlier.
People like me who have spent time around industrial labs and workshops get used to seeing warning labels on bottles and barrels. There’s wisdom in that caution. Even chemicals that sound innocent—take household bleach—can mess you up in the wrong circumstances. Many of us learned the hard way in high school: splash a spot on your jeans, get that hole. Acids, solvents, and even some simple salts demand respect, not just a shrug and a glance.
Exposure adds up, and not every risk jumps out right away. Maybe you breathe something in, or maybe a spill soaks through your gloves. I once thought a quick scrub would solve everything after a minor lab spill, but my skin burned for hours. Some chemical hazards don’t need dramatic exposure—chronic, low-level contact can harm lungs, liver, or even the brain. Benzene, for example, was used carelessly for decades before people understood its link to leukemia.
The Harvard T.H. Chan School of Public Health reports that over 13 million workers in the United States use chemicals every year. Occupational Safety and Health Administration stats show chemical exposures cause thousands of preventable injuries and illnesses. The World Health Organization connects certain solvents and heavy metals to cancer, kidney failure, or developmental problems. Chemicals can damage living cells through inhalation, skin contact, or ingestion. Sometimes the risk hides until emergency rooms fill with patients who only thought they were “handling things just fine.”
Labels offer a starting point. Some folks ignore warnings, thinking a little splash won’t hurt, but the label reflects science, accident reports, and sometimes funeral statistics. The chemical safety data sheet (SDS) usually sits on a shelf, collecting dust, but every time I’ve read one, I learned something new—especially about reactivity or side effects I didn’t expect.
People in labs and factories wear gloves, goggles, and sometimes full-body suits—not because they’re scared, but because no one enjoys skin rashes or breathing problems. Proper ventilation doesn’t just clear the air; it stops fumes from building up, and it saved my lungs more than once. Spill kits and eyewash stations seem like overkill until the day someone drops acid—or ammonia catches you by surprise.
Some chemicals really are safe in ordinary settings. Water doesn't need a hazmat team, and table salt isn't going to put you in the hospital. Still, it’s easy to get comfortable and think, “I’ve done this a hundred times,” right before disaster hits. Back in college, a friend mixed bleach and ammonia in a cleaning frenzy. After the paramedics left, nobody made that mistake twice.
Approaching chemicals with a level head has saved my skin—and others’. Asking “Is this hazardous to handle?” isn’t just bureaucracy; it has real stakes. Looking up credible sources, wearing basic protection, storing bottles in the right spot, and not eating a sandwich mid-cleanup won’t just keep you off the injury report. It lets you work with confidence, knowing you’re not gambling your health on a shortcut.
Whether it’s formaldehyde, paint thinner, or a plastics solvent, “safe enough” isn’t a good guess. Trusting expertise, reading safety sheets, and learning from those who’ve seen mishaps happen keeps both hands steady and your lungs working the way nature intended.
Working in a lab or a workshop often means grappling with substances you’ve only seen in textbooks. Looking up whether something dissolves in water, ethanol, acetone, or oil turns into a real headache when the chart in front of you doesn’t match the batch on hand. You notice tables listing dozens of options but never quite the nuance you’d need. Why? Solubility isn’t just technical trivia—it changes the outcome of experiments, the stability of a medicine, the safety of a process.
Once, faced with a stubborn organic compound during a university summer job, I needed it to blend in for a reaction. Water wouldn’t cut it. Ethanol took too long. I wasted half a box of gloves and more lab time than I’d care to admit. That experience taught me: real-world chemistry depends on solubility like cooking relies on the right oil for the pan.
Most think about solubility in terms of “polar dissolves polar, non-polar dissolves non-polar,” but reality shrugs at these sayings. For every rule, there’s some weird exception. Take acetone: usually an all-star for organics, but some bulky molecules refuse to go near it. Or look at DMSO—famous for dissolving almost anything, useful for difficult cases, also requiring extra safety steps because it brings things straight through the skin.
Solubility depends on a few real factors: the specific chemical structure, the presence of functional groups (hydroxyl, amine, carboxyl groups), temperature, and pH. For instance, adding a methyl group might make a compound shift from “sort of water liking” to “nope, strictly organic solvent.” Chemists have watched a small tweak in structure take a molecule from being soluble in ethanol to only dissolving in chloroform.
Google Scholar and databases like SciFinder list countless solubility data points, but they don’t always help when your issue is temperature difference or a slightly changed structure. Textbooks suggest broad guidelines—“esters like ethyl acetate,” “alkaloids like chloroform”—but in practice, every batch of a compound seems slightly different. It matters because manufacturers, pharmacy techs, or students mixing up a buffer don’t want “maybe.” They need “yes, at this temperature, in this solvent, this amount dissolves.”
Problems pile up when product datasheets or online specs gloss over real solubility data. Some suppliers will just list “soluble in organic solvents,” but that’s about as helpful as saying a food is “edible.” If you’re in product development, missing out on key solubility info can ruin a run. If you need to avoid toxic solvents, knowing your compound is only soluble in dichloromethane forces you to weigh health versus efficiency.
One path forward: researchers reporting their full set of solvent-solubility tests in open-access formats, not hiding it behind paywalls. Another: using modern tools—machine learning models or advanced calculators—can help predict solubility with higher accuracy, shaving off trial-and-error time in busy labs. I’ve worked alongside teams that turned to computer models when old references fell short, and they often came away with a working solution in hours, not weeks.
Solubility isn’t just a bullet point on a datasheet. It’s the difference between a process that runs smooth and one that stalls. Those doing the dissolving need straight answers, built on strong experience and tested facts. Better data, better transparency, and more sharing of real lab results will make the question “What solvents is this compound soluble in?” less of a guessing game.
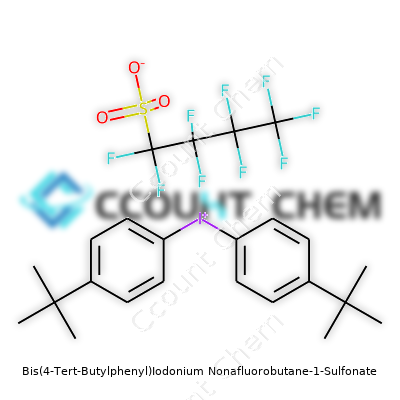
| Names | |
| Preferred IUPAC name | bis[4-(tert-butyl)phenyl]iodonium 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate |
| Other names |
4-tert-Butylphenyl[4-(tert-butyl)phenyl]iodonium nonafluorobutanesulfonate Bis(4-tert-butylphenyl)iodonium nonaflate Bis(4-tert-butylphenyl)iodonium nonafluorobutanesulfonate |
| Pronunciation | /ˈbɪs fɔː ˈtɜːt ˈbʌtɪl ˈfiːnɪl aɪˈɒdəʊniəm ˌnəʊnəˌflʊəroʊˈbjuːteɪn wʌn ˈsʌlfəneɪt/ |
| Identifiers | |
| CAS Number | 1221931-47-6 |
| Beilstein Reference | 2736741 |
| ChEBI | CHEBI:137162 |
| ChEMBL | CHEMBL3984097 |
| ChemSpider | 27548246 |
| DrugBank | DB11024 |
| ECHA InfoCard | 01a7a9dc-6a8c-42f2-aa6b-57f7a79f68de |
| Gmelin Reference | 1050284 |
| KEGG | C16197 |
| MeSH | D000072637 |
| PubChem CID | 129700982 |
| RTECS number | GF1921000 |
| UNII | X8891B0XOZ |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID90862901 |
| Properties | |
| Chemical formula | C28H34F9IO3S |
| Molar mass | 678.43 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.44 g/cm3 |
| Solubility in water | Insoluble |
| log P | 4.3 |
| Vapor pressure | Vapor pressure: < 0.00001 hPa at 20 °C |
| Acidity (pKa) | -3.7 |
| Magnetic susceptibility (χ) | -75.8 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.600 |
| Viscosity | 700 cP (25°C) |
| Dipole moment | 3.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | null |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H317, H319, H411 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P333+P313, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | NFPA 704: "1-1-0 |
| Flash point | > 235°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Bis(4-tert-butylphenyl)iodonium hexafluorophosphate Bis(4-tert-butylphenyl)iodonium triflate Diphenyliodonium hexafluorophosphate Diphenyliodonium tetrafluoroborate Bis(4-methylphenyl)iodonium hexafluorophosphate |