Walk into any chemistry lab with a history shelf, and you’ll see compounds like aniline-2-sulfonic acid have shaped more than just textbooks—they've nudged entire industries. Chemists in the late 19th century, sparked by the successes of William Perkin and the sensational birth of synthetic dyes, started hunting for ways to tame and transform aromatic amines. Early accounts talk about chemists sulfonating aniline using concentrated sulfuric acid, trying not to scorch the material or themselves, waiting impatiently for purple or blue hues to dot their flasks. The push for brighter, longer-lasting dyes led to more precise methods, which also revealed properties that attracted more attention from pharmacologists and materials scientists. The progress from trial-and-error beakers to industrial tanks echoes in the raw practicality and risks of scale-up.
Aniline-2-sulfonic acid stands as a white to buff crystalline powder, recognized for its knack at shuttling sulfonic acid groups onto aniline and opening up a pathway to dozens of dye intermediates and specialty chemicals. You’ll find this compound coursing through the supply chains of textile colorants, agrochemicals, and pharmaceuticals, not just as a raw material but as a stepping stone for larger molecular frameworks. Its value comes from straightforward chemistry paired with stubborn reactivity—a quality that never goes out of style in industrial synthesis.
Firm, gritty crystals that dissolve easily in water—especially when hot—give aniline-2-sulfonic acid a hands-on familiarity in the lab. Solubility in polar solvents makes it handle well during extractions and purifications. Laying out the structure, its amine and sulfonic acid sit in ortho positions, tweaking both the boiling point and acidity. Melting usually happens around 250°C with slow decomposition. The molecule keeps its integrity under moderate conditions but will give up the sulfonic group to stronger nucleophiles or during directed modifications. Technicians have learned to recognize the telltale faint smell and the quick way it picks up moisture, reminding you to cap that bottle tight.
Factories and suppliers mention purity above 98% for dye intermediates and analytical needs. Bulk material often carries labels flagging the CAS Number 88-21-1, the chemical formula C6H7NO3S, as well as the correct hazard codes for irritancy, environmental effect, and disposal requirements. Packaging meets UN and GHS standards with batch numbers and dates for traceability. Safety Data Sheets keep users in the loop about everything from flash points to the right material for sample scoops.
Across years of experience, manufacturers have stuck with sulfonation as the key route—pouring aniline into a cooled bath of concentrated sulfuric acid and keeping everything at just the right temperature to steer the product to the 2-position. Too much heat or too strong an acid, and side-products take over, bringing trouble during downstream separations. Some have tried tweaking with fuming acids or reusing spent acid, learning quickly how yield and color shift with each small variable. Purified by repeated reprecipitation or crystallization from water, the product can reach technical grades needed for demanding work in dye chemistry or small-volume custom synthesis.
This compound plays well with both acids and bases. Its amine group takes on acylation or diazotization, opening access to azo dyes or triazine derivatives. The sulfonic acid group itself resists reduction but encourages straightforward coupling to form sulfonamides or metal complexes. Laboratories watch closely for the possibility of double sulfonation, which shifts functional behavior and sometimes frustrates control over synthesis. Given the right partners, aniline-2-sulfonic acid punches above its weight—marking a starting point for more intricate scaffolds in material science and pharma.
Old-school catalogs and modern databases list this product under names like ortho-aminobenzenesulfonic acid, 2-aminobenzenesulfonic acid, and sulfanilic acid (though this last name sometimes confuses with the para-isomer). Depending on the suppliers and the market, you'll see references like 2-Anilinesulphonic acid, o-Anilinesulfonic acid, or even technical names relating to its use in specific dyestuffs.
Personal experience has shown how handling this compound, even in well-ventilated spaces, means gloves, goggles, and quick-acting spill response. Though not the most hazardous sulfonic compound, aniline-2-sulfonic acid causes irritation of the skin, eyes, and mucous membranes. Chronic exposure can sensitize workers, so regulators set occupational exposure limits and require continuous training. Good practice means closed handling systems, routine checks for airborne dust, and disposal of waste in line with local and international regulations. Manufacturing lines run best with clear labeling, scheduled maintenance on exhausts, and ready-to-use spill kits near storage areas.
The story of aniline-2-sulfonic acid threads through more than just dye bathtubs. Chemists tap into its versatility when producing acid dyes for wool, silk, and nylon—a small nudge in pH, and the color sticks like it belongs there. Pharmaceutical companies convert it into drug intermediates; for instance, its transformation to sulfa drugs illustrates how industrial chemistry shifts toward practical ends. Outside of medicine, agrochemical makers count on its stability and reactivity, slotting it into crop protection products and analytical reagents. In research labs, it pops up when scientists model new catalysts or proteins, taking advantage of the molecule’s duality—both nucleophilic and electrophilic points on the same ring.
Current efforts focus on cleaner, more efficient synthesis, driven partly by demands for greener chemistry and partly by cost control. Enzyme-mediated routes, recyclable solvents, and continuous processing all get tested in pilot labs and upscaled factory settings. Computational chemists now model reaction pathways, predicting outcomes before anyone commits a flask of expensive reagents. New analytical methods tighten quality control, sharpening the line between desired product and impurity. Research teams frequently check in with applied scientists, making sure modifications at the molecular level translate into better product performance—whether for deeper dye shades or lower residue in pharma actives.
Decades of studies confirm that inhalation or skin exposure brings irritation, sometimes sensitization, and rare but possible systemic toxicity if mishandled in high doses. Chronic exposure risks, like carcinogenicity or reproductive effects, remain under constant review as workplace norms update and analytical tools improve. Researchers keep an eye on downstream effects, knowing that wastewater from dye works and pharma plants sometimes still dumps enough load to shift aquatic ecosystems. Treatment plants run toxicity screens, while industrial health officers check biomarkers in exposed workforces—circling back with preventive measures based on sound, evidence-backed science.
Looking ahead, as clean chemistry and process intensification become staples, production of aniline-2-sulfonic acid will likely move to platforms that reuse both solvents and energy. Regulation tightens year by year, so only plants that blend efficiency with strict compliance will keep market share. Applications should widen, too, beyond traditional dyes—showing up in niche pharmaceuticals, advanced coatings, and even electronic materials. Researchers are already using machine-learning predictions and automated labs to propose modifications for stability or function, chasing after precise color control or catalytic power. With markets demanding higher purity and sustainability, this modest molecule still draws attention—not for what it did in the past, but for the roles it can play as technology and regulation evolve.
Factories churn out all sorts of chemicals, but not every compound gets much attention outside the lab. Aniline-2-sulfonic acid might not make headlines, but it plays a big part in products people use each day. If you've ever enjoyed a bright-colored T-shirt or read a book with crisp, black ink, you may have crossed paths with the results of this compound.
Industry experts often keep a close eye on how raw materials shape entire product families. In the world of dyes, small changes to a molecule can transform the shade or durability of color. Aniline-2-sulfonic acid, for instance, helps make azo dyes, which account for almost two-thirds of all dyes made worldwide. These compounds find their way onto fabric, leather, and even paper. Without this acid, many popular colors would fade fast, bleed, or lose brightness after a few wash cycles.
Azo dyes stand out for their strong color and stability against light and washing. Aniline-2-sulfonic acid acts as a foundation, building the vivid reds, oranges, and yellows seen on textiles. The chemistry behind these colors matters because poor dye quality leads to more waste if products get thrown out early, and can also raise environmental concerns from excess chemical runoff.
Outside the world of fashion and print, aniline-2-sulfonic acid pops up in pharmaceutical labs. Some medications rely on chemical reactions that need a helper or starting point. This compound helps create certain antibiotics and analgesics by providing a key structure during synthesis. Small changes to a molecule can influence how a drug behaves in the body, so precision at this stage sets the tone for quality down the line.
In the rubber industry, manufacturers look for methods to protect rubber from breaking down over time. Aniline-2-sulfonic acid enters the mix, leading to chemicals that slow aging and keep tires or conveyor belts useful for longer stretches. Quality here keeps roads safe and reduces the pileup of spent materials at landfills.
With all these upsides, the use of aniline-2-sulfonic acid raises a real challenge: safe handling and disposal. Runoff can stir up trouble for water sources if left unchecked. Studies link exposure to aniline-based compounds with health concerns; strict workplace rules and containment strategies limit the risk. I remember hearing from a chemist in a textile plant who wore extra layers of gloves and double-checked all containment lines because a single spill could pollute the wastewater and threaten fish or farming nearby.
More chemical plants now track emissions closely, use closed-loop water systems, and recycle what they can. While not perfect, these steps help make sure the same substances that brighten our clothes and keep roadways safe don’t darken the land or our health.
Aniline-2-sulfonic acid stands at the center of several industries. Its uses touch everything from what we wear to road safety and healthcare. A responsible approach to its production and disposal gives hope that people can keep reaping its benefits without leaving a mess for future generations. Raw chemistry shapes daily life, and smart practices rooted in science and care ensure that its impact stays positive.
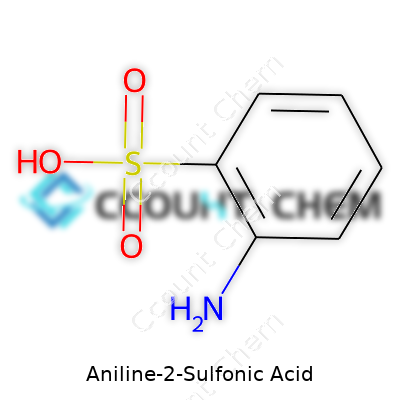
Chemistry can feel intimidating, yet every chemical tells a story through its formula. With aniline-2-sulfonic acid, the curiosity starts right at its structure. Its formula is C6H7NO3S. Nothing fancy, just carbon, hydrogen, nitrogen, oxygen, and sulfur, yet those elements connect in ways that keep entire industries running.
Most people don’t wake up thinking about chemicals like aniline-2-sulfonic acid, but its presence is felt nearly everywhere. In the dye industry, it helps bring color to fabrics on the racks downtown. That red shirt? There’s a good chance a compound like this got involved somewhere along the line. Chemists have spent over a century figuring out which molecules help color stick to fibers, resist sunlight, and keep their brightness.
I remember visiting a textile plant as a student. The smell and the colors felt overwhelming, but behind all that lies careful science. Mixing the right chemicals in precise amounts keeps products safe for skin, reliable in washing machines, and bright through the seasons.
Mislabeling a substance doesn’t just hurt a process; it hurts people and brands. Picture a batch of synthetic dye going wrong. An incorrect formula could mix harmful byproducts or waste expensive ingredients. Public trust isn’t won by keeping formulas secret but by sharing them precisely, then proving what’s in every jar and barrel. Regulators, factory workers, and customers all rely on clear, trusted declarations.
Aniline-2-sulfonic acid isn’t something that sits on the kitchen shelf. It isn’t edible and needs respectful handling. Sulfonic acids can burn or cause allergic reactions. My uncle, a safety officer at a chemical plant, kept stacks of safety data sheets on hand. He’d say, “You don’t wing it with acids.” Proper labeling, robust safety training, and the habit of reading every label matter more than any single piece of equipment in the lab.
The straightforward formula, C6H7NO3S, helps scientists and manufacturers identify the compound without confusion. Beyond factories, the education system can do a better job making these substances less mysterious for students. If more classrooms spent time with hands-on demonstrations and open Q&A about chemical safety, fewer accidents would happen down the road.
Tools for tracking and verifying chemical identities have improved. QR codes on containers, digital databases for safety data, and mobile apps for lab workers turn complicated data into clear, everyday decisions. At home and globally, demanding open, honest formulas lets buyers and businesses avoid cuts and shortcuts that put safety on the line.
There’s a kind of quiet confidence in knowing exactly what goes into every product. Whether someone handles aniline-2-sulfonic acid directly or simply enjoys a shirt dyed with its help, everyone benefits from formulas spelled out, shared, and respected from creation to consumption.
Aniline-2-sulfonic acid makes its way into dyestuff manufacturing, pharmaceuticals, and sometimes lab research. I’ve sat at a lab bench with its white powder sitting in a bottle, and even then I can’t ignore the label warnings. Many chemicals, especially those with an aniline backbone, should never be dismissed as harmless — handling this compound safely means respecting its risks, not just memorizing guidelines from a textbook.
The substance carries health risks. Breathing in its dust irritates airways and feels similar to being caught in a room full of strong cleaning agents. Once it gets on the skin, there’s often an itch or rash that follows, which can escalate without proper washing. Eyes exposed to small amounts get red and sore almost immediately. Its reputation for causing methemoglobinemia—a blood disorder that robs the body of oxygen—deserves real concern, not just a passing mention in a safety seminar.
In factories making dyes, it often ends up all over gloves, benches, and the soles of shoes. Some workers don’t notice symptoms until they’re blue around the lips or short of breath, all because a bit of powder slipped past a mask. The OSHA guidelines single this chemical out for a reason: just a little careless exposure, repeated day after day, adds up. Chronic exposure links to problems in the liver, spleen, and definitely the blood. Even in small operations, stories crop up about “the guy who went home early” because he forgot to check his respirator. Facts show clear chronic health risks. The compound also lingers in soil and water when not handled correctly, so communities around chemical plants can face long-term risks without direct contact.
Engineering controls and personal protective equipment really make the difference. I always trusted nitrile gloves and splash goggles, not out of paranoia, but because it only takes one accident to drive the lesson home. Fume hoods should run by default. Good ventilation needs to become basic practice everywhere this compound gets used.
Some workplaces take shortcuts by using cheap dust masks, but published toxicology studies show how inadequate those are. Real protection includes a fit-tested respirator where airborne dust is possible. Routine accidents get avoided by tight protocols: closed containers, designated spill kits, and fast cleanup of any powder before it tracks to non-lab areas. Regular skin checks and quick access to clean running water keep minor issues from turning worse.
Industry leaders could push for alternatives in the dye sector, seeking out less hazardous starting materials. That direction needs targeted research and honest comparisons — not just picking new molecules with softer names but actually verifying lower risks. For now, regulators and process engineers bear extra responsibility for setting stricter workplace limits and supporting thorough training.
Safer disposal options can’t be afterthoughts. Waste must go into specialized containers, and company policies should incentivize return-and-recycle programs for empty packaging. Sharing incident reports among firms could prevent copycat accidents. Public access to safety data and real exposure numbers helps workers and residents alike make informed decisions. In my experience, the more open a workplace is about health risks, the less likely large-scale harm becomes.
Aniline-2-sulfonic acid finds its way into dye manufacturing, pharmaceuticals, and lab research. The stuff can irritate skin and eyes, and its dust or vapors trigger sneezing fits and coughing spells. Keeping it dry, cool, and away from the wrong chemicals doesn’t only matter for shelf life — it keeps people safe and avoids costly cleanup headaches.
Humidity breaks this acid down. I’ve opened old drums that turned from powder to sticky lumps, all because they spent months in a damp storeroom. So, keep the container sealed and in a spot where moisture can’t creep in. Once the powder starts clumping up, it spells trouble for downstream processes and tells you something’s wrong with storage.
Temperature swings also play tricks on chemicals. A warm storeroom shortens lifespan, and if things get hot enough, labels fade or peel and plastic containers begin to sweat or deform. I recommend shaded, well-ventilated spaces — avoid storing anywhere near machinery that radiates heat. Firmer containers, preferably glass or designated chemical-safe plastics, keep everything stable.
A big mistake I’ve seen in warehouses is putting acids close to bases or flammable solvents. One overturned pail, and you have a major chemical reaction – with fumes or, in worst cases, fire. Aniline-2-sulfonic acid should be miles away from storage for strong alkalis, oxidizers, or materials prone to combustion. Color-code shelving or mark zones to reinforce the message, especially with changing staff or new trainees.
Every chemical must have a readable label with clear instructions and hazard symbols. Yet, I’ve pulled faded labels from mystery bins and found hand-written notes instead of manufacturer print. That kind of shortcut breeds accidents. Chemical-handling guidelines and the exact date received on each drum prevent confusion and make tracking expiration easier. Digital inventory systems do even better by sending reminders about old stock.
Spills and exposure still sneak up on careful teams. Stock eye wash stations and chemical spill kits close by. Train everyone to recognize symptoms of exposure, since some people may react more quickly than others. Fast response prevents bigger medical complications and calms nerves in the warehouse.
Data from chemical safety boards show that incidents involving aniline compounds often come down to poor segregation and forgotten containers. Many companies think once a drum gets parked on a shelf, the job’s over — but routine inspections and clear housekeeping rules catch leaks and signs of container breakdown. In my own work, the best-run facilities hold monthly walkthroughs and keep logs for each chemical’s storage spot.
Extra effort spent on safe storage proves cheaper than dealing with an accident or regulatory fine. Investing in proper containers, waterproof labeling, and assigning clear responsibility for inventory pays off every time. People trust their workspaces more and productivity actually climbs when hazard risks fall. I’ve seen places with clear storage zones and a tight inventory count have fewer lost raw materials, fewer sick days linked to exposure, and no big incidents on the record.
Aniline-2-sulfonic acid usually shows up as a pale to white solid. That color sometimes sits closer to beige if there’s a touch of impurity or if the batch picked up a bit of moisture. You’ll find it in crystalline or powder form — both feel a little gritty between your fingers, not slick or waxy like some chemicals. If you leave it out in open air, the crystals might clump a bit, absorbing water from humidity.
This compound doesn’t stand out by smell. Unlike aniline, which has a sharp, fishy odor that lingers, the sulfonic acid version keeps things much more toned-down. I’ve handled it in labs, and it won’t hit your nose unless you really lean in. That’s a relief because dealing with stronger-smelling chemicals for hours gets tiring fast.
Solubility always comes up. Aniline-2-sulfonic acid dissolves in water, but not as fast as table salt. Warm water speeds up the process — you’ll notice cloudy swirls that eventually even out. Its solubility in organic solvents runs low. So, if you try stirring it into acetone or ether, it tends to settle out. That plays into safety, since this keeps the chance of sudden reactions lower in multi-step syntheses or in crowded work spaces.
The melting point sits well over 200°C. During chemical processes, that helps avoid unplanned melting or decomposition, which I’ve seen ruin batches in high-temperature setups. Once, I made the mistake of not monitoring the heat closely: the crystals didn’t melt, but beyond a certain point, you get decomposition, with an unpleasant change in appearance and smell.
The acid character of this compound stands out in practice. If you splash a bit of sodium carbonate in, expect fizzing. You’ll get a pH drop in water — it acts much stronger as an acid than plain aniline. This impacts how you choose your equipment, especially if you store it for weeks. Glass handles it fine, but cheap metals or containers with slight corrosion end up staining or pitting.
Most of the aniline-2-sulfonic acid goes toward making dyes. The sulfonic acid group helps the molecule stick to fibers better. That keeps colors from washing out quickly. Textile labs like the white crystals because they signal a pure starting point for vibrant hues. In my experience, trace contamination throws off the final color and can mess up batches worth thousands of dollars. Careful control in storage and transfer is not just about safety but also keeping profits steady.
Handling safety needs attention, too. Even though its odor seems harmless, it’s not smart to underestimate any sulfonic acid. Bare skin gets irritated. Spills call for gloves and eye protection. Inhalation isn’t much of a risk compared to volatile solvents, but I always run the exhaust fan to be safe. Accidents often come from ignoring small steps, like skipping the respirator during big weighing jobs. Emergency showers and eye-wash stations give peace of mind, but prevention works far better.
Consumers and workers both benefit when manufacturers dial up transparency about purity and handling instructions. I’d like to see clearer labeling that spells out what impurities are present and what percentage water content to expect. Technicians with limited budgets often make equipment last longer than it should. Extra guidance and lower-cost testing kits help those teams avoid unnecessary risk, keeping both employees and final products safer. Good ventilation and clear procedures cut accidents and make working with this compound more predictable.
| Names | |
| Preferred IUPAC name | Benzenamine-2-sulfonic acid |
| Other names |
2-Anilinesulfonic acid o-Aminobenzenesulfonic acid Orthobenzenesulfonic acid 2-Aminobenzenesulfonic acid |
| Pronunciation | /ˈæn.ɪ.liːn tuː ˌsʌlˈfoʊ.nɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 88-21-1 |
| Beilstein Reference | 1040257 |
| ChEBI | CHEBI:27860 |
| ChEMBL | CHEMBL22322 |
| ChemSpider | 14904 |
| DrugBank | DB03755 |
| ECHA InfoCard | 100.010.016 |
| EC Number | 246-376-1 |
| Gmelin Reference | 7615 |
| KEGG | C01762 |
| MeSH | D000882 |
| PubChem CID | 8596 |
| RTECS number | BW7400000 |
| UNII | 7LI3H75T1E |
| UN number | UN2585 |
| CompTox Dashboard (EPA) | DTXSID2020489 |
| Properties | |
| Chemical formula | C6H7NO3S |
| Molar mass | 173.20 g/mol |
| Appearance | White to pale gray powder |
| Odor | Odorless |
| Density | 1.485 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.31 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.03 |
| Basicity (pKb) | 3.68 |
| Magnetic susceptibility (χ) | -46.5 × 10⁻⁶ cm³/mol |
| Dipole moment | 2.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -259.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1515 kJ/mol |
| Pharmacology | |
| ATC code | null |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 195°C |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2230 mg/kg |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Aniline-2-Sulfonic Acid: 5 mg/m³ |
| REL (Recommended) | 1.25 mg/L |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Aniline Sulfanilic acid p-Aminobenzenesulfonic acid o-Aminobenzenesulfonic acid Benzene sulfonic acid 2-Nitroaniline |