Ammonium sulfamate didn’t arrive out of nowhere. For over a century, chemists and industrialists hunted for solutions that tackled invasive plant growth and managed troublesome waste in cost-sensitive ways. This chemical emerged through work in the early 1900s when weed control started moving from hand labor to systemic science. Post-WWI agriculture and public works needed safer alternatives to caustic acids and unreliable manual methods. Its popularity grew on farms and public lands that needed no-nonsense solutions for brambles, nettles, and stumps. By the 1930s, ammonium sulfamate punched above its weight as a manageable, water-soluble herbicide — and later, its uses stretched far beyond fields, moving into paper mills, fire retardant industries, and laboratory benches.
A bag of ammonium sulfamate powder looks dull, but its reach extends from home gardens to industrial pulp digesters. Produced as a free-flowing, white crystalline solid, it handles easy transport and measures out neatly for field use. It carries the legacy of materials that demand both practical handling and chemical consistency. Farmers use it to clear ground for new plantings, and city workers rely on it to keep invasive species from chewing through park landscapes. Its mild odor and quick solubility in water let users create precise sprays or pour-on treatments — but don’t be fooled, this isn’t a hobbyist’s toy. That same bag finds use for wood preservation or, at modest concentrations, in firefighting to reduce combustibility of buildings near wildland-urban interfaces.
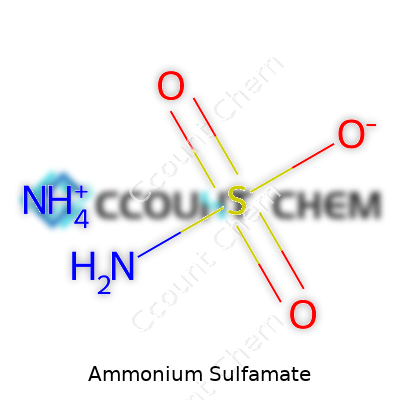
Pure ammonium sulfamate boasts the formula NH4NH2SO3. Its melting point hovers near 131°C, well above room temperature, giving it storage stability across most climates. Dissolving it in water takes little effort. The resulting solution slides into application gear without clogging or leaving gritty residues. Its solubility, about 75g per 100 ml at 20°C, surpasses many older herbicidal salts. Chemically, the compound stays stable under ambient conditions, but heated above its melting point or exposed to strong bases, it breaks down to liberate ammonia and sulfur oxides — the whiff of ammonia always lets you know there's a spill. This chemistry enables targeted actions and controlled reactions in the field as well as in industrial processes.
A typical technical label lists ammonium sulfamate content at 99% purity or higher, with chloride and nitrate impurities capped well below 1%. Each package carries batch numbers, manufacturing dates, and standardized hazard icons for irritancy and environmental risk. Labeling regulations require clear guidance for personal protective equipment and first-aid measures, spelling out use restrictions for watercourses and protected habitats. Standards from agencies like the EPA, REACH, and OSHA set documentation for safety data, exposure limits, and disposal routes. Labels warn against mixing with strong acids or bases and detail storage needs: dry, sealed, and out of direct sunlight, far from children and animals. For the professionals handling municipal weed eradication or forest fire retardation, these details back up every shift.
Industrial-scale ammonium sulfamate comes from direct reaction of ammonia with sulfamic acid in water. Factories feed ammonia gas into large, jacketed reactors while steadily adding sulfamic acid, controlling temperature to avoid runaway fumes or excess heat. The mixture cools, and the dissolved ammonium sulfamate crystallizes on standing, sometimes aided by chilling. Filtration and careful drying produce the familiar free-flowing, granular powder. Experience with chemical processes taught me long ago — if the temperature creeps up too much, side reactions leave unhelpful byproducts. Producers check the product using titration or ion chromatography, discarding off-spec material. Small-scale synthesis in labs can follow the same method, though batch sizes shrink to beaker level and ventilation matters more than profits.
In basic water, ammonium sulfamate acts as a weak acid, ready to lose hydrogen and generate ammonium ions. When exposed to strong bases or heat, it decomposes — letting off ammonia, nitrogen, and sulfur dioxide. Lab chemists value this breakdown for prepping controlled-release nitrogen sources. Rarely, its structure allows derivatization, but commercial applications focus on its simple, rugged form. The salt doesn’t combine with most metals under ambient conditions, which lessens corrosion risks in application gear. It doesn’t go inert, though. In paper pulping or fiber bleaching, it reacts gently, enabling lignin removal without tearing cellulose structures. Municipal weed control teams count on this soft chemistry during stump destruction — and seasoned workers always flush application equipment, since leftover residues can scale up, gum parts, or corrode fittings.
Chemists learned early on that ammonium sulfamate shows up under several names. A purchase order could carry the name "ammonium amidosulfate," "ammonium sulfamate," or "ammonium aminosulfonate." Gardeners might spot it in stores as "Amicide," "Aminosulfuric Acid Ammonium Salt," or legacy brands like "Weedol" in Europe. My time in municipal infrastructure introduced me to drummed shipments marked as "AS-50" and “Weed Wand.” Fire safety teams often see "fire retardant ammonium salt" as a catch-all, so checking CAS numbers (7773-06-0) clears up confusion. These names reflect decades of evolving uses, regulations, and local terms — but the chemistry hasn’t changed.
Safe handling demands respect for skin and airways. Ammonium sulfamate powder irritates eyes and nose, especially during windy application or spill situations. My own experience during roadside applications reminds me — even mild compounds need gloves, goggles, and dust masks. Industry standards from regulatory bodies, including OSHA and the European Chemicals Agency, specify engineering controls and first-aid responses. Emergency plans must cover skin and eye rinsing, spill containment, and safe waste disposal. Operators transport it in sealed packaging, avoiding high heat or open flames, and color-coded storage bins. Fire departments noted that burning ammonium sulfamate releases toxic gases, so even small fires involving this chemical demand SCBA and evacuation. Waterways, garden ponds, and protected wetlands get flagged for exclusion zones — regulators build buffer zones to guard native wildlife. Ongoing safety checks and operation reviews sharpen company procedures and lower risk for everyone in the field.
Ammonium sulfamate carves out a broad range of uses. It performs as an herbicide for brushwood, bramble, and invasive weed removal. Homeowners turn to this material for clearing persistent moss and algae in patios, driveways, and fencing. Agricultural teams broadcast it to prepare land for planting or manage boundary strips. Forestry workers dump it into drilled holes in fresh stumps to rot them out, turning stubborn hardwoods into compostable mulch. Paper industry engineers load reactors with ammonium sulfamate to bleach pulps for high-quality stationery and book paper. In construction, fire safety codes in some jurisdictions allow its use as a fire retardant treatment for timber, textile, or thatch roofing. Lab researchers value purified grades for a gentle nitrogen source or as a mild sulfonation reagent. Personal experience tells me its versatility hinges on correct dosing and strict label reading — overapplication can scorch soil or linger in run-off, pushing the need for good stewardship.
Ongoing research works at two main fronts. Scientists develop less toxic, more biodegradable options that maintain effectiveness without long-term soil impact. Universities investigate ways to blend ammonium sulfamate with co-formulants, aiming to expand spectrum while reducing off-target effects. Environmental chemists track its persistence, breakdown products, and movement through soil and groundwater using new tracing methods. In industry labs, technicians chase efficiency in pulp bleaching, adjusting process temperatures and concentrations to squeeze out better output or cut reaction times. Fire safety engineers experiment with new application systems for wildland buffer zones and flammable structures. Across research, the hunt for balance persists — users need effective treatments without harming the natural web or exposing workers to hidden risks. R&D centers now field feedback loops with field teams, taking hard-won experience from farms, parks, and paper mills back into development so new formulations meet real-world chores.
Toxicologists studied ammonium sulfamate since its debut. Acute exposure typically causes mild irritation, though large doses ingested by pets or livestock bring nausea or vomiting. There’s no robust evidence linking it to cancer or birth defects in routine uses, but chronic overexposure may damage soil microflora or aquatic life. European regulatory reviews in recent decades flagged overuse risks, prompting tighter restrictions and buffer zone requirements. Safety clearances reflect moderate hazard — safe for use if handled properly, risky if misused or dumped near water or food sources. This is the same balance found in many practical chemicals, so rigorous enforcement and safety training become crucial. Recent risk assessments encourage using it late in the season, away from bloom time to shield pollinators, and keeping applications off windy days that spread drift beyond target zones. Long-term studies explore breakdown speed in differing soils, charting routes for even safer use.
Future trends point to focused applications and greater control. Technological advances in spray calibration, ground imaging, and remote sensing make precision application standard — less wasted product, fuller coverage, lower environmental impact. Research teams dig deep into designing slow-release versions for stump treatment, hoping to extend rot without repeated dosing. Regulatory shifts and climate challenges mean everyone from farmers to city engineers needs alternatives tailored for local flora and water protection. There’s talk of engineered microorganisms that can metabolize runaway residues, turning a chemical problem into a biological solution. Some industry groups weigh whether new fire codes or renewable building materials will reduce reliance on chemical retardants — but for now, ammonium sulfamate stays in demand for spots where hand labor, flame, or bladed equipment won’t do. No easy fix competes with the straightforward results this compound brings when used with care and respect for its strengths and limitations.
Ammonium sulfamate pops up in places where people face tough weeds, roots, and the never-ending battle to keep certain plants from taking over. Gardeners, landscapers, and groundskeepers rely on it to control brambles, ivy, and other stubborn growth that laughs at weaker solutions. I’ve seen allotment holders swear by this stuff, especially when nothing else seems to stop invasive roots. It's not just about killing off what you don't want—it's about reclaiming a patch of land that the nettles or brambles tried to seize for good.
The white, granular form dissolves easily in water. People dilute it into a solution and apply it directly to cut stumps or leaves. If you’ve ever tried to clear old hedgerows or dig unwanted saplings, that quick action can feel like a godsend. The chemical disrupts the way plants grow, so the leaves wither and the stubborn root systems don’t keep coming back.
One big reason ammonium sulfamate gained popularity comes down to price and safety compared to harsher weedkillers. Glyphosate and similar chemicals often hit headlines over health and environmental worries. Ammonium sulfamate breaks down into natural components—ammonia, nitrogen, and sulfate ions—when the soil’s microorganisms get hold of it. That appeals to anyone who wants to avoid lingering toxins, especially on plots used for growing food later.
Data from several UK allotment studies show lower residue risks where ammonium sulfamate treats perennial weeds. Results like those factored into its continued use by hobbyists and professionals who prize less persistent options. Over years of community gardening, I’ve spoken with folks who transitioned from old-school herbicides to this alternative for exactly that reason—peace of mind about next season’s crops.
Forestry teams, councils, and railways often reach for ammonium sulfamate not just in one-off gardening jobs, but as a solution for repeated clearance. For power lines or tracks where unchecked saplings and fast-growing weeds can cause safety headaches, this tool gets results. It doesn’t tie workers up for multiple applications through the season, and the breakdown process means equipment and crews don’t have to worry about accidental contamination down the line.
Firebreaks are another area where you’ll find this chemical in play. By controlling vegetation in zones that could spark wildfires, it helps form defensive strips. Experienced wildland managers know regular manual clearing can suck up a huge amount of local government budget—tools like ammonium sulfamate make those budgets stretch further without locking land into decades of chemical buildup.
Some regions have pulled back on ammonium sulfamate for home use. Not because it’s especially toxic to people, but because every chemical can build up if misused. Australia, for example, still allows its use in professional settings, while the UK has tightened the rules over the last decade. This makes sense—responsible handling matters with anything that can alter plant or soil chemistry.
Safety data shows low toxicity for humans and animals compared to standard weedkillers, but that doesn’t mean it’s “organic” or harmless. The real solution lies in common sense: safety gloves, strict attention to label instructions, and thinking ahead about runoff or nearby crops.
With weeds adapting fast to conventional products, the need for alternatives rises every year. Ammonium sulfamate’s reputation—in my experience—revolves around its ability to finish tough jobs quickly and fade away without long-term ugliness. For people restoring gardens, protecting infrastructure, or fighting invasive plants without a long list of residual worries, this chemical still earns its space on the shelf. It’s not magic, but it gives people more control without harming tomorrow’s harvest.
I’ve worked with gardeners for years, especially those frustrated by invasive weeds creeping into beds and hedgerows. Ammonium sulfamate often pops up in conversations as a weed control tool. It’s quick to dissolve in water, easy to apply, and reportedly breaks down into harmless compounds. But once the container comes out, people start asking, “Is it safe for my pets? Will it ruin my favorite plants?” These are smart questions—gardeners and farmers have to balance necessity with care for their environment.
Ammonium sulfamate acts as a selective herbicide, hitting tough brambles, nettles, and woody stems harder than your grass or perennial flowers. The science behind it seems reassuring. Research shows that it quickly breaks down in the soil, forming ammonium and sulfate ions, both common in compost and fertilizers. The UK’s Health and Safety Executive once approved it for use, labeling it with less concern than more persistent herbicides.
I always remind people that “breaks down quickly” doesn’t mean totally risk-free. Spraying it on green foliage means absorption into roots and stems. Over-application risks harm to non-target plants, especially those with delicate new foliage. Using it under trees, for example, can accidentally damage shallow-rooted species. I’ve noticed this myself when overzealous weedkillers leave yellowing patches under old stone walls.
Most home gardeners want to invite birds, bees, and hedgehogs—not scare them away. The good news: ammonium sulfamate shows low toxicity to mammals and birds according to the World Health Organization. There’s no sweetness to tempt pets, and unlike slug pellets or rodent poison, it hasn’t triggered waves of vet visits in my community. But keep animals off freshly treated areas until they dry, just in case. Wet paws or noses may pick up residue, and exposure in small animals never brings peace of mind, no matter the statistics.
Fish and amphibians need special mention. Runoff can be tricky, especially near ponds and streams. Even biodegradable chemicals don’t belong in fresh water. I’ve learned to avoid spraying close to the edge and use physical barriers to trap drips or leaching.
Reading the label counts—precision matters. Spot treatment with a hand sprayer works better than soaking whole patches, especially in mixed beds. Never use it on food crops or herbs. I see safer, older tools like hand weeding and mulching still holding their place. Some prefer vinegar or boiling water on paths, both harmless to wildlife, though less effective on deep roots.
Personal experience says moderation works best. Chemicals solve problems, but never replace observation. Watching how insects drift back, how new growth emerges after a targeted spray, gives more feedback than any guidebook. If in doubt, skip the chemical route—usually, patience and persistence win in the long run.
Ammonium sulfamate rarely ranks as dangerous in the home garden. Still, it deserves the same respect you’d give bleach or fertilizer—careful handling, measured amounts, and a backup plan for any mistake. The best gardeners I know always aim for fewer chemicals and more hands-on care. Trust your experience, balance new science with old wisdom, and keep an eye on plants and animals sharing your patch of land.
Ammonium sulfamate has built its reputation as a reliable brushwood killer and general herbicide for gardeners and land managers who want control over stubborn weeds, brambles, and invasive plants. Many folks look for a way to get rid of deep-rooted plants that just seem to keep coming back no matter how many times they are cut down. This is the product many seasoned gardeners turned to before a wave of new chemicals entered the market.
I’ve watched people pour or spray chemicals over their garden without much thought, expecting overnight miracles. Product labels might get ignored. But from my own experience, skipping instructions often means wasted product, or worse, injured plants that you actually want to keep. Ammonium sulfamate sets itself apart because it targets unwanted woody weeds right at the source.
Plants like Japanese knotweed and bramble can seem nearly impossible to eradicate. Even after cutting them back again and again, those roots just do not quit. Applying ammonium sulfamate directly to freshly cut stems gets right down into the root system. I’ve seen neighbors try to treat large areas by spraying, only to have the plants reappear after a few months. Going after the target plant’s fresh wound with a paintbrush or sprayer usually gets better results.
Timing often makes the biggest difference. Brambles, docks, nettles, and bamboo all respond better when the chemical gets in soon after the first cut. Early summer or late spring works well, while the plant is actively moving sap. Applying in dry weather means the solution stays and does its work instead of washing away. I know it can be tough to wait for the right window. It’s tempting to blitz everything right away, but patience helps.
Dilution rates matter, too. Look for the guidance on the container. Mixing too strong can burn the target area without moving enough product to the roots, while weak solutions just don’t cut it. I’ve walked through community gardens where patchy application left green shoots between burnt-out stems. Measured mixing often avoids this. A five percent solution usually covers most woody weeds.
I’ve felt the sting of losing a few prized rose bushes because of accidental drift. Cover the plants you want to keep and avoid breezy days. Paint carefully, make sure run-off doesn’t trickle down to roots of nearby favorites. Wearing gloves and eye protection keeps you safe, since ammonium sulfamate can sting if it touches bare skin or eyes.
Ammonium sulfamate breaks down into fertilizer after it does its job, so it doesn’t stick around in the soil for years. That makes it more approachable for community gardens and people with pets or kids running around. But using chemicals should always come with responsibility. Try physical barriers, repeat hand removal, or mulching as follow-up methods. Over the years, I’ve learned that patience, a little bit of science, and care for the land will pay off more than shortcuts. Responsible use of ammonium sulfamate can clear land now and set you up for healthier growth next season.
Ammonium sulfamate shows up in places you might not expect, from garden sheds to industrial warehouses. Most folks who spend time with this chemical use it to keep weeds out of sight or to help in composting. Yet, even though it might sound routine, the risks sneak up on people who treat it carelessly. I've handled plenty of chemicals in my gardening days, and I’ve learned there's a reason for every safety rule on a sack or bottle.
It stings when it hits exposed skin, in the same way as other strong salts. One careless grab and skin can turn red or even blistered, especially if you forget gloves. Eyes feel it worse. If it gets in, you’re looking at a burning, tearing mess that could leave real damage. Every time I work with ammonium sulfamate, I lean on tight-fitting gloves and goggles. The CDC and other health agencies support this approach because once that powder finds moisture, it moves fast.
Dust clouds in sunlight look harmless, but breathing in this stuff isn’t smart. Fine particles drift right into the nose or throat. For folks with asthma or allergies, this can start a bad day. Even for those without breathing problems, it's not wise to let it settle in your lungs. A dust mask isn’t overkill—it's a must. Choose one rated for fine particles, not just basic sawdust, and you’ll sidestep most problems.
I’ve seen bags tossed in sheds next to dog food and sacks of grass seed. That’s asking for trouble. Moisture turns ammonium sulfamate into a liquid that eats through metal and stains wood. Heat might ramp up chemical changes, which brings out toxic fumes. A dry, cool spot away from pets, kids, and sunlight keeps risks low. Keep containers sealed up tight—think screw-top plastic, not a half-tied bag. After each use, wash hands and scrub any spills right away.
Out in the garden, some folks forget that mixing it with other stuff could jump-start dangerous reactions. Adding bleach or other cleaners doesn’t just waste money; it brews up toxic gas. Use clean, dedicated tools and stick to diluting it with plain water, as the instructions suggest. Clean all the gear thoroughly—don’t let leftover crystals sit on shovels or sprayers, where they could corrode metal or get picked up later by accident.
It pays to know your first aid here. Eye contact means rinsing for more than fifteen minutes, not just a quick splash. Skin contact? Soap, water, and toss those gloves or clothes out of reach of others. If dust ends up in your mouth or lungs, see a doctor without delay. Quick action limits serious harm. I keep the local poison control number handy, just in case.
Dumping leftovers down the drain or into the yard will make things worse for waterways and soil. Most communities run hazardous waste drop-offs a few times a year, and should take ammonium sulfamate without issue. Check local regulations, because rules shift from place to place. It's worth the trip to keep this out of the wrong stream or landfill.
Each of these steps shows respect—for your own health, for the people sharing your home or workplace, and for the environment. Accidents cost more than just money; they clamp down on trust and bring trouble that lingers much longer than an afternoon of gardening or cleaning ever should.
Gardeners, landscapers, and some DIY users have turned to ammonium sulfamate for a long time. It’s probably best known for killing tough weeds and helping rot down stubborn tree stumps. For most people, the search starts at local garden centers, agricultural suppliers, or specialist shops online. Some bigger hardware stores also stock it, though not always right on the shelf. I once tracked it down at a rural agricultural co-op. That said, availability isn’t what it used to be. In plenty of places, you’re more likely to find it behind the counter or on a restricted shelf.
E-commerce sites—Amazon, eBay, and a few European gardening shops—carry ammonium sulfamate. Retailers usually label it clearly as a weedkiller, compost accelerator, or stump remover. Shipping rules pop up depending on where you live, as it sits in a gray area between common weedkillers and chemicals that need closer watching. Some countries count it as a controlled substance, especially in large amounts.
A few years back, ammonium sulfamate was still sold over the counter all across the UK and Ireland. Things changed after tighter rules kicked in across Europe, partly because of environmental pressure and concern about chemical misuse. In the United States, the Environmental Protection Agency hasn’t given it the nod as a registered pesticide. This means you won’t see it for sale as an herbicide at places like Home Depot or recognized U.S. garden chains. Most American buyers hunt for it through specialty chemical suppliers, and almost always for non-pesticide reasons.
In Australia and New Zealand, regulations vary by state, with some treating it as a standard garden chemical with few hoops to jump through, and others setting clear restrictions or outright bans. The differences reflect local approaches to environmental risks and how much trust officials place in do-it-yourself users.
Rules around ammonium sulfamate didn’t appear out of nowhere. Just as with other garden chemicals, overuse or misuse can harm more than intended. Residue might seep into the water table, linger in the soil, or throw off natural cycles if used carelessly. Some countries also keep an eye on its potential use in unsafe activities far from gardening.
From my experience, regulators weigh public safety above convenience. On one hand, gardeners lose a reliable weedkiller and composting boost. On the other, open sales could bring risks few people consider until things go wrong. It gets tricky for folks who rely on ammonium sulfamate for legitimate landscaping or forestry projects.
People who need ammonium sulfamate for genuine purposes stand a better chance by contacting licensed suppliers and checking national databases of approved chemicals. Local garden clubs or horticultural coordinators can often clarify if you’re playing by the current rules or headed for trouble.
If you’re locked out by new regulation, switching to approved alternatives usually makes sense. There are newer, greener stump and weed treatments—acetic acid solutions or pelargonic acid—that do a decent job without legal headaches. Compost fans can still use old-fashioned nitrogen boosters like urea, avoiding regulatory minefields.
The take-home: Know the rules in your country before you go searching. Demand for ammonium sulfamate isn’t going away, but neither is the pressure to keep public spaces and waterways safe from risky chemicals.
| Names | |
| Preferred IUPAC name | Ammonium sulfamate |
| Other names |
Ammonium amidosulfate Ammonium sulfamate Amidosulfonic acid ammonium salt Ammoniumsulfamate Ammoniumsulfamid Sulfamic acid ammonium salt |
| Pronunciation | /əˈməʊniəm ˈsʌlfeɪmæt/ |
| Identifiers | |
| CAS Number | 7773-06-0 |
| Beilstein Reference | 358741 |
| ChEBI | CHEBI:29773 |
| ChEMBL | CHEMBL1546 |
| ChemSpider | 21438 |
| DrugBank | DB11410 |
| ECHA InfoCard | 03c3b0c1-22ef-43b2-b5e8-d6974135fcb4 |
| EC Number | 205-549-7 |
| Gmelin Reference | 5276 |
| KEGG | C14657 |
| MeSH | D018242 |
| PubChem CID | 23668281 |
| RTECS number | BT0525000 |
| UNII | P5T8P556AA |
| UN number | UN2967 |
| CompTox Dashboard (EPA) | DTXSID5023865 |
| Properties | |
| Chemical formula | NH4SO3NH2 |
| Molar mass | 114.12 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.75 g/cm³ |
| Solubility in water | Highly soluble |
| log P | -2.77 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 1.0 |
| Basicity (pKb) | 7.15 |
| Magnetic susceptibility (χ) | −47.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.396 |
| Viscosity | 1.82 cP (20 °C) |
| Dipole moment | 1.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -773.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -644.8 kJ/mol |
| Pharmacology | |
| ATC code | V03AX03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H318 |
| Precautionary statements | P210, P261, P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P330, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Autoignition temperature | 630 °C (1166 °F; 903 K) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD₅₀ oral (rat): 2,150 mg/kg |
| LD50 (median dose) | 1310 mg/kg (rat, oral) |
| NIOSH | RN8180000 |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Sulfamic acid Ammonium sulfate Ammonium nitrate Potassium sulfamate |