Chemists first started to take notice of Ammonium 2-Hydroxyethanesulphonate in the early waves of modern organic chemistry research. The pursuit to produce biodegradable and biocompatible sulfonates for industrial and laboratory use gave this compound a role in many experimental procedures. Thinking back to how research opened up during the 20th century, folks were searching for alternatives to harsher alkali or strongly acidic compounds. The arrival of the hydroxyethanesulphonate salts brought a chance to balance reactivity with gentler physical profiles, a breakthrough for labs battling both purity requirements and tightening environmental standards. What made it stand out was its relatively simple synthesis, reliable solubility, and behavior in aqueous settings. As scientists worked through decades of trial and error, this salt found its place in several catalogs, growing from a specialty chemical to a reliable staple for advanced applications.
Ammonium 2-Hydroxyethanesulphonate steps in as a versatile ionic compound with an appealing combination of solubility and stability. In practical terms, it offers a strong sulfonic group joined to a two-carbon chain that ends with a hydroxyl group, counter-balanced by ammonium. What sets it apart from other similar compounds is the easy way it handles water, quickly forming clear solutions that open doors for both chemical reactions and formulation work. This compound starts off as a white crystalline powder, lacking the pungent scent of related amines, making it easier to handle in tight laboratory quarters. The features aren’t just theoretical—the ease in measuring, dissolving, and storing matter a lot for anyone who’s worked at a fume hood or bench for long hours.
On the bench, you see a free-flowing crystalline substance, white with no visible clumps, and low in hygroscopicity compared to some ammonium salts. Chemically, it dissolves in water at room temperature and creates a solution neutral or just slightly acidic in pH, depending on the batch and water source. The molecular weight usually lands around 143 grams per mole, and it resists decomposition under normal conditions unless exposed to strong bases or acids. Those who’ve spent time preparing stock solutions can appreciate how cleanly it blends, without leaving telltale residues on glassware—a relief for anyone who remembers grappling with poorly soluble salts. Its melting point sits on the higher side of 200°C, and you don’t get troublesome dust or volatility issues, which helps in safe handling and accurate weighing.
Suppliers often ship Ammonium 2-Hydroxyethanesulphonate in well-sealed plastic drums or foil-lined bags, each marked with lot numbers, purity ranges, expiration dates, and hazard pictograms. Specifications usually demand a content of at least 98-99%, with trace metals and moisture levels tightly controlled—because even minor impurities can cause headaches in sensitive syntheses or analytical work. Labels outline recommended storage—cool, dry, away from strong acids or bases—and highlight key hazards, such as mild irritancy if inhaled or in contact with skin. MSDS sheets go a step further, calling attention to fire safety, though this compound resists ignition unless heavily contaminated. In research labs, accurate, up-to-date labeling guards against accidents and ensures teams stick to consistent product quality—something I’ve learned matters more than anything for reproducible results.
Preparation usually starts with neutralizing 2-hydroxyethanesulfonic acid (sometimes called isethionic acid) with concentrated aqueous ammonia. The process means adding ammonia slowly to the acid solution, with constant stirring to avoid localized heating or splattering. Monitoring the pH and temperature closely as ammonium ions form, the solution shifts from a slightly viscous texture to clear and runny. Crystallization requires slow evaporation or cooling, followed by filtration and washing with cold ethanol, which scrubs away side impurities and leaves behind pure product. Experienced lab workers know to dry the crystals under vacuum or in a low-temperature oven, watching out for over-drying, as this can make some ammonium salts degrade or clump. That extra care keeps purity high, which in my own lab work has meant fewer downstream problems.
Ammonium 2-Hydroxyethanesulphonate stands out for its chemical flexibility. The sulfonic acid group can undergo esterification, forming sulfonate esters used for more hydrophobic applications, while the hydroxyl group makes it ideal for coupling reactions. In practice, it blends nicely with other ammonium salts or can switch out its counterion in ion-exchange setups, producing a family of related sulfonates. Those working on surfactant or buffer research have used it to tweak pH characteristics without introducing strong acids. It doesn't oxidize readily, which adds to its appeal for sensitive syntheses. I’ve found it especially helpful where a balance between water solubility and ionic strength is needed, such as in catalyst stabilization or measuring enzyme activity.
Navigating chemical catalogs can get confusing, as Ammonium 2-Hydroxyethanesulphonate also goes by names such as Ammonium Isethionate, or company-specific codes like AIEtS or AMS. Chemists ordering from different vendors might spot the CAS number 2935-88-0, which helps to sort through the forest of similar salts. Synonyms may reflect slightly different hydration levels or manufacturing processes, but the chemical backbone stays the same—a lesson quickly learned through both hands-on work and countless order forms. Reliable tracking across synonyms means fewer order mistakes and smoother research projects—a simple but crucial lesson from years of lab management.
Strict attention to safety prevents problems down the line. Touching or inhaling the dust can cause minor discomfort, so gloves and light respiratory protection make sense during weighing or transfer. Although not a flammable substance, it should be kept away from strong oxidizers and sources of open flame due to possible decomposition of accompanying materials. Adequate ventilation, eyewash stations, and up-to-date safety training form the backbone of responsible lab operation. Waste solutions go into designated chemical collection, with pH checks before pouring into containers labeled for ammonium salts. In my own experience, facility audits and emergency drills pay off when unexpected spills or exposure incidents crop up—doing things by the book saves time and trouble for everyone involved.
One of the strong points for Ammonium 2-Hydroxyethanesulphonate comes from its use as a catalyst or buffering agent in biochemical reactions, where its stability and mild acidity make it valuable for controlling reaction rates. Folks working in pharmaceutical analytical labs use it to solubilize certain drug substances without flooding solutions with sodium or potassium ions. Textile and cosmetic industries have turned to it as a mild surfactant and antistatic agent; its chemical stability means fewer surprises in otherwise complex formulations. Water treatment teams sometimes reach for it due to its high solubility and low toxicity, especially in specialty ion-exchange or polishing filters. Those who've spent days troubleshooting process lines value predictable compounds like this—one less variable to worry about means more reliable production.
Ongoing research circles around new applications and greener synthesis routes for Ammonium 2-Hydroxyethanesulphonate. Chemistry teams are always searching for ways to skip harsh solvents, increase yield, and cut waste. Polymer scientists study its behavior in chain termination or as a hydrophilic modifier for smart gels and membranes. In the field of analytical chemistry, adjustments to column buffers or sample stabilizers push the boundaries for sensitive assays. Academic partnerships with industry have spurred innovation in the synthesis of new derivatives, aiming for compounds with improved biodegradability or tailored solubility. Years of open sharing between universities and industrial teams underscore how collaboration leads to better, safer, and more sustainable solutions.
Every new use prompts more questions about human and environmental safety. Toxicity research so far places Ammonium 2-Hydroxyethanesulphonate as low risk in typical lab or consumer settings. Studies in rats and aquatic organisms reveal mild irritancy at high concentrations, but little chronic toxicity. Medical research points out the benefits of fast renal excretion, which limits accumulation in the body. Environmental monitoring looks at breakdown in wastewater, revealing that this compound doesn’t persist or bioaccumulate, unlike many other sulfonates. Regular review of safety data, paired with sound handling practices, keeps risks to a minimum—a pattern long embedded in my own and my colleagues’ laboratory routines.
As industries shift toward sustainability, chemists see room for expanded use of Ammonium 2-Hydroxyethanesulphonate in biodegradable formulations, medical device coatings, and next-generation cleaning compounds. The ongoing move away from heavy metals and harsh surfactants creates new markets for milder, more environmentally sound agents. Research continues into refining synthesis methods—such as using waste ammonia from industrial processes or greener sources of isethionic acid. Governments and agencies push for closed-loop manufacturing, prompting teams to develop better recycling and waste-management protocols for these compounds. From firsthand experience, staying ahead in the development and regulatory curve ensures continued access to effective, safe chemicals like this one. The lessons drawn from years in the field prove that reliable compounds—backed by transparent research—will keep supporting both innovation and better stewardship of health and the environment.
Look at modern industry, and you’ll notice a range of chemical helpers involved in everything from cleaning up water to powering batteries. Ammonium 2-hydroxyethanesulphonate sits in that lineup, working mostly behind the scenes. My own background in biochemistry made me pay close attention when a compound quietly supports several processes.
This chemical pops up in water treatment plants, playing a role as an antiscalant and cleaning agent. Managing scale, or mineral buildup, becomes tougher for municipal water systems year after year. Engineers look to this ammonium salt for its ability to break down calcium deposits and reduce clogs. Keeping machinery and pipes clean keeps water flowing safely and helps local governments save money on repairs. I've seen city treatment teams switch to more benign additives like this one, replacing harsher chemicals with lower toxicity.
Engineers who build batteries often face a tricky balancing act—making sure each cell provides reliable power without breaking down or catching fire. Ammonium 2-hydroxyethanesulphonate earns its place in some liquid electrolyte formulas. By supporting ionic conductivity and stabilizing mixtures, it lends a hand to those seeking safer, longer-lasting batteries. Tech companies hunting for better rechargeable batteries look for materials that aren’t too flammable or toxic, and this salt usually checks those boxes.
Not all cleaners are created equal. This salt comes in handy for industrial cleaning blends, especially those aimed at sensitive equipment or electronics. Its mild profile pairs well with surfactants, which helps remove soil and residue without causing corrosion. Factories dealing with steel or glass equipment get products that do the job without wrecking the surfaces. I’ve seen it help manufacturers cut down on downtime caused by failed cleaning routines.
While the chemical offers value across several sectors, some uncertainty remains about broader public safety. Many specialty chemicals fly under the radar, so full research on environmental persistence and long-term toxicity sometimes lags behind practical use. Regulators need data to back up claims of low risk. I’ve heard water tech professionals call for more independent testing so that regulations keep up with real-world conditions.
There’s also the matter of responsible sourcing. Many ammonium salts are commodities, and a steady supply keeps prices down. Yet global supply chains and variable quality present challenges. In my experience, transparency helps customers trust that they’re getting what they pay for—not just a substitute that barely meets the minimum.
Producing safer, more efficient chemicals will always matter. Facility managers and formulators ask for ingredients that don’t harm workers or neighborhoods. Policymakers can make a difference by supporting better reporting requirements and encouraging green chemistry initiatives. Make sure companies reveal sourcing, and fund more environmental testing. These steps give communities a chance to enjoy the benefits of chemistry without unexpected fallout.
Ammonium 2-hydroxyethanesulphonate often shows up in industrial and research settings, mainly as a buffering agent or part of more complex chemical recipes. Chemists and technicians who deal with it see a white crystalline powder and wonder if it deserves more caution than the usual fray of laboratory chemicals.
Safety sheets from manufacturers lay out basic concerns with clarity. You won’t find claims of high toxicity, nor does this compound belong in the class of corrosive, highly hazardous, or acutely toxic chemicals listed by regulatory bodies like OSHA or the European Chemicals Agency. Still, everyday chemistry experience teaches that comfort with routine shouldn’t turn into carelessness.
I’ve handled ammonium 2-hydroxyethanesulphonate a few times, mostly as a buffer system for analytical chemistry projects. Gloves, goggles, and lab coats were always on. Once, a student skipped gloves, thinking the material looked about as threatening as table salt. Minor irritation on the skin cleared up quickly, but it was a reminder that “not dangerous” isn’t the same as “harmless.”
This story isn’t unique. Most cases in chemical environments echo this: Skin or eye contact leads to mild irritation at worst, according to toxicological data. Inhalation, a less likely risk due to its low volatility, can irritate respiratory passages, just as most dusts do. Ingesting the powder would probably trigger stomach upset and little more, though that’s no excuse for lax behavior in the lab.
Many chemicals, including ammonium 2-hydroxyethanesulphonate, don’t grab headlines for danger, yet still ask for respect. It’s about culture, not paranoia. Every time a team gets complacent, forgetting PPE for “mild” compounds, the odds of accidents creep up. Over time, tiny exposures or dozens of small cagey habits break down the reliability and trust built in good laboratories.
Longstanding data from the CDC confirms that repeated exposure to “minor irritants” in labs account for a large share of reported incidents year after year. Each event may seem negligible, but they add up — lost productivity, discomfort, maybe even compounding allergies or sensitivities over careers spent in the lab.
Training makes a difference. Seeing chemical safety not as hoops to jump through, but as rituals that let everyone return home healthy, gives real meaning to the routine. Quick labels, up-to-date data sheets, and regular team meetings keep best practices from slipping into the background.
Switching from old, casual habits to using fume hoods, disposable gloves, and splash goggles for all powders — no matter how mild — dramatically lowers risk. Lab culture sets the tone: If veteran staff review practices openly, newcomers follow their lead. Even casual chemicals pick up a respect they probably warrant, given the unpredictable nature of the work.
Based on what we see in the lab, ammonium 2-hydroxyethanesulphonate falls on the safer side of the spectrum. It won’t burn skin or poison air, but smart handling protects everyone. Safety succeeds when it’s more about habit than hazard ratings — and that’s true for every white powder, not just the notorious ones.
I keep coming back to the same theme: details shape everything. That runs through chemistry, regulation, safety, and, believe it or not, ethical business. With Ammonium 2-Hydroxyethanesulphonate, also called ammonium isethionate, there’s a unique number that should draw attention: the CAS number 7076-60-1. Some might shrug at a seven-digit label, but this tiny code keeps labs clear-headed, helps prevent costly mix-ups, and keeps dangerous errors at bay.
A lot of my time working with ingredient reviews has shown me the value of precision. In the chemical trade, relying solely on common names leads to serious confusion. Ammonium 2-Hydroxyethanesulphonate sometimes crops up under trade names, regional titles, or slightly varied spellings. The CAS number removes doubt; there’s only one 7076-60-1. That clarity slopes into everything, from safety data sheets to customs forms and technical documentation.
Consider the process in product development. Cosmetic labs depend on chemical purity and traceability. Swapping one compound for a similar-sounding substitute—think sodium for ammonium isethionate—can force recalls or destroy months of research. That punishment isn’t just theoretical: in 2018, a personal care manufacturer paid out thousands fixing a problem traced to a small technical mistake with ingredient numbers. That painful episode keeps me vigilant to this day.
The safety sheets for ammonium isethionate only match up when the compound is correctly identified. Tens of thousands of chemicals roll through global markets every month. Emergency workers or regulators trying to interpret garbled paperwork risk real danger. Incorrect labeling slows down emergency responses, sometimes stalling critical treatment. For every downstream worker who checks formulas or audits records, seeing “CAS 7076-60-1” spells out accountability and trust.
The European Union’s REACH regulations, and similar rules across Asia and North America, tie their rules to CAS numbers. Importing a batch of ammonium isethionate to an EU plant means paperwork must match exactly, with mistakes drawing fines or blockage. The same story unfolds in the cosmetics and food industry, where ingredient identity faces legal scrutiny almost daily.
Every chemist, product manager or buyer who handles Ammonium 2-Hydroxyethanesulphonate runs into the need for clean data. I now urge newcomers in chemicals to double-check every reference—be it labels, digital files or supplier invoices—and to verify CAS numbers before hitting “approve.” Just last year, I watched a small startup sidestep major trouble by catching a supplier with a misprinted number before shipping. That moment proved more than luck; it showed the worth of experience and rigorous training.
Transparency in ingredient sourcing makes reordering easy but keeps the bar high for safety and compliance. Consistency in using recognized identifiers like CAS isn’t bureaucratic—it’s practical risk management. The more attention we give these details, the fewer headaches for everyone, from bench chemists to quality officers and consumers. This number, 7076-60-1, ends up being a technical bridge connecting science, law, and public trust.
Handling chemicals in any lab or industrial space shapes more than just daily routines—it protects health, resources, and budgets. Ammonium 2-hydroxyethanesulphonate, mostly known for its role in industrial and laboratory processes, asks for careful storage. Ignoring the basics doesn’t just slow down workflow; it opens the door to accidents, waste, and even regulatory headaches.
Many underestimate the problems that poor storage brings. This compound won’t explode on its own or catch fire as quickly as some better-known reactives. Still, like any salt that pulls in moisture, it clumps up fast if left out in the open. That’s money down the drain, since you can’t mix dried-up lumps into a reaction without weird results. It’s common sense—leave salt in a humid room, expect a sticky mess.
In my own experience working around various chemical stocks, any bottle that absorbs water quickly grabs attention. A storeroom that smells musty or feels muggy turns pristine powders into bricks. Managers start to grumble about “losses,” but the root cause traces back to skipping basic preventive steps.
A simple dry shelf won’t cut it. Airtight containers make a difference—you can’t go wrong with sturdy screw-tops and an inner plastic seal. In offices and universities I’ve dealt with, glass keeps out moisture better than thin plastic, especially for anything hygroscopic. Silica gel packets, little bags that hide inside the cap, earn their keep more often than folks admit; swapping them out once they turn pink saves entire batches from going bad.
No need for unusual temperatures. Normal room conditions work fine so long as the area doesn’t swing wildly between hot and cold. Hot storerooms lead to condensation, and that’s all it takes for a granular powder to fuse into a solid mass. Dark spaces work better because certain chemicals break down under bright sunlight, and nobody wants to guess if the white powder they ordered last month still does the job today.
Thrown-together shelves spell problems for anyone who’s had to sweep up accidental spills. Organizing chemicals by compatibility stops the domino effect: one knocked-over bottle can mean costly decontamination or—worse—a reportable spill. Ammonium 2-hydroxyethanesulphonate behaves, so long as it keeps away from oxidizers and strong acids. Color-coded bins and good records take confusion out of the equation. In one older facility I visited, a switch from messy handwritten labels to printed barcodes trimmed down mistakes by half in only a few months.
The best chemical cabinet can’t fix a lack of training. Staff turnover in labs and manufacturing happens all the time. Newcomers guess, toss bottles anywhere, or leave caps loose. Regular refresher sessions push out bad habits and refresh the basics. Written instructions, checked and signed, give everyone the confidence to do things right. Online trackers here help with shelf-life monitoring; it keeps product fresh and guides disposal before waste becomes a safety risk.
No lab or plant enjoys unscheduled maintenance or wasted material. Storing Ammonium 2-hydroxyethanesulphonate in an airtight, labeled, dry, and cool environment keeps things simple. Care now saves resources later—clean chemicals mean smoother work, fewer headaches, and a safer environment for those working the front lines of industry and science.
Ammonium 2-hydroxyethanesulphonate draws attention for several reasons. With the growing demand for safer alternatives in cleaning products and industrial applications, this compound often shows up on the list of options worth considering. Its roots go back to typical salt chemistry; it blends a sulfonic acid with an ammonium group, plus it has an extra hydroxy group standing out. That leads to an interesting combination of properties, both physically and chemically.
This compound takes on a white, crystalline powder form, which means it’s not going to create visibility or staining challenges. It usually dissolves pretty well in water, letting workers in labs or factories mix it up quickly without stubborn batches stuck at the bottom. If you've ever worked with solutions where full dissolving seems impossible, you’ll know why this matters. Ease of mixing saves time and keeps things predictable.
Its melting point falls within a comfortably moderate range for handling and shipping. The material doesn’t break down under standard ambient temperatures. Heat it up far enough and it does start to decompose, but that doesn’t happen during daily tasks or in normal storage settings. Moisture in the air won’t set it off—it resists clumping due to humidity, which reduces storage headaches.
Chemically, ammonium 2-hydroxyethanesulphonate draws water, which means it behaves as a hygroscopic material. That can make a difference during storage, where tight lids become important. Its ionic structure helps it hold up well in solution, supporting chemical reactions in places like buffer mixtures or electroplating baths.
Because its sulfonic acid group sits behind many detergency effects, workers in cleaning or brightening applications pay close attention. The hydroxy group can improve solubility and influence how the compound interacts with other ingredients, sometimes boosting performance for stain removal or acting as a dispersant. These interactions help the product stand out from simpler ammonium salts.
Any worker who handles chemicals pays attention to the potential hazards. This compound doesn’t pack the punch of some more aggressive acids or bases, but it still deserves respect. Direct contact could cause mild irritation to skin or eyes. Inhalation isn’t recommended, but a properly ventilated lab or worksite handles that risk. I remember always checking the safety data sheets myself and making sure gloves and eye protection fit comfortably—small steps, but worth it to avoid regrettable moments.
Environmental impact plays a bigger and bigger role in chemical selection. Ammonium 2-hydroxyethanesulphonate breaks down relatively easily in water treatment plants, leaving behind less of a long-term footprint than phosphates or persistent organic pollutants. The balance between performance and responsibility feels crucial, especially as regulations become stricter and customers demand cleaner practices.
Looking at possible improvements, producers could experiment with packaging options that limit exposure to humidity. Automated handling systems cut down on direct employee contact. Simple steps like these drive better safety and results on the floor. The way this compound bridges performance and responsibility often makes it a steady pick when you want dependable chemistry with fewer trade-offs.
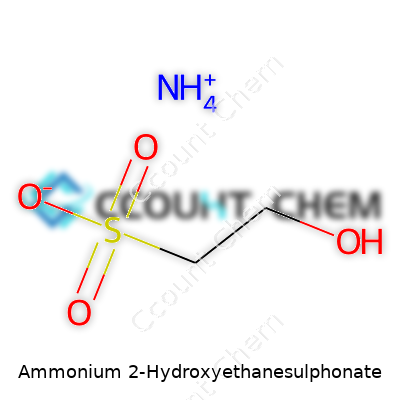

| Names | |
| Preferred IUPAC name | Ammonium 2-hydroxyethane-1-sulfonate |
| Other names |
Isethionic acid ammonium salt Ammonium isethionate Ammonium 2-hydroxyethane sulfonate Ammonium isethionate salt |
| Pronunciation | /əˈmoʊniəm tuː haɪˈdrɒksiˌeθeɪnˈsʌl.fəˌneɪt/ |
| Identifiers | |
| CAS Number | 10042-31-6 |
| 3D model (JSmol) | `/pdb/jmol/jsmol/php/jsmol.php?model=CC(O)CS(=O)(=O)O.NH4` |
| Beilstein Reference | 1109070 |
| ChEBI | CHEBI:37960 |
| ChEMBL | CHEMBL2103836 |
| ChemSpider | 85581 |
| DrugBank | DB13866 |
| ECHA InfoCard | ECHA InfoCard: 03-2119969270-36-0000 |
| EC Number | 225-392-5 |
| Gmelin Reference | 113145 |
| KEGG | C19614 |
| MeSH | D000635 |
| PubChem CID | 2723826 |
| RTECS number | BP8484000 |
| UNII | 1M8B8N41PC |
| UN number | UN3261 |
| CompTox Dashboard (EPA) | DTXSID3021328 |
| Properties | |
| Chemical formula | C2H9NO4S |
| Molar mass | 125.17 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -3.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.8 |
| Basicity (pKb) | pKb ≈ 4.75 |
| Magnetic susceptibility (χ) | -6.1×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.421 |
| Viscosity | Viscous liquid |
| Dipole moment | 7.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 226.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -906.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -969 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | S01XA30 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P362+P364 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2564 mg/kg |
| NIOSH | RN8810000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not established |
| Related compounds | |
| Related compounds |
2-Hydroxyethanesulfonic acid Ethanolamine Ammonium sulfate Sodium 2-hydroxyethanesulfonate Potassium 2-hydroxyethanesulfonate |