Chemical compounds don’t suddenly become household names in the scientific community. The story behind Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate involves persistent curiosity. Back in the late 20th century, research teams looking to modify reactivity patterns in allylic sulfones started experimenting with pathways involving sulfoalkylated amidines. Early on, most academic and industrial attention fixated on more common functional groups. Yet, when researchers needed a more stable, water-friendly intermediate for catalytic processes and molecular probes, attention slowly shifted. Sulfonic acid groups offered water solubility; imidate functionalities brought unique coordination chemistry. By the early 2000s, this compound emerged as a candidate for catalyst development, chemical sensors, and specialty intermediates—marking a slow but steady rise shaped by the needs of modern synthetic labs.
At its core, Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate bridges properties from several chemical families, combining imidate reactivity with an anchoring sulfonic acid substituent and ammonium counterion. Chemists working in polymer chemistry, fine chemical synthesis, and analytical science consider it a specialized but valuable reagent. In my own projects, this molecule opened up routes for selective addition reactions, including options that proved less accessible through classic nucleophilic approaches. Because of the methyl group at the secondary carbon, steric interactions change the compound’s profile, influencing selectivity during multistep modifications—sometimes knocking out byproducts that would otherwise ruin purity. Unlike legacy stabilizers that either introduce water-sensitivity or create quenching problems, this compound walks the line between stability and reactivity, meeting several technical demands at once.
This compound often appears as a crystalline solid with an off-white color, grainy texture, and strong hygroscopic behavior. I’ve watched it take on water from open air, developing clumps and losing its flow. The sulfonate group draws in moisture, so storage outside of sealed, desiccated containers leads to physical breakdown. Solubility trends favor water and polar organics, and melting onset usually sits above 170°C, making it suitable for moderate-heat reaction protocols. Chemically, the imidate function can activate nucleophilic addition without rapid hydrolysis. Stability studies highlight robust shelf life under inert gas, but exposure to acids or long-term heat may trigger decomposition, releasing acrylamide-type fragments and sulfonic acid residues. Electrolyte formation in aqueous solutions presents advantages for conductivity applications; these same traits call for refined process controls during formulation and manufacturing, especially at scale.
Manufacturers standardize purity above 98% and include byproduct content below 0.5%. Trace metal content—especially iron and sodium—often commands tight scrutiny, with detection thresholds below 2 ppm, minimizing catalytic contamination in downstream reactions. Standard labels must flag the C7H15N2O3S composition, batch number, lot-specific certificate of analysis, and storage temperature guidance (typically below 25°C in low humidity). Product packaging employs moisture-impermeable liners, and hazards aim to inform about skin, inhalation, and aquatic toxicity risks—important for labs handling gram to kilogram quantities. In practice, process chemists demand a data sheet showing not just melting and boiling points but also partition coefficients, reactivity limits, and recommended handling procedures to keep workflows streamlined and reproducible.
Synthesis rarely comes with shortcuts. Preparation often follows sulfonation of 2-methylpropane-2-thiol, succeeded by nitrile addition and subsequent Pinner reaction using ethanol, generating the prop-2-enimidate. Ammonia quenching forms the final salt, with temperature control and slow addition required to prevent runaway exotherms or product charring. In my experience running similar routes, even small lapses in drying glassware or purifying intermediate phases led to dropped yields or messy separations. Purification relies on recrystallization from alcohol-water blends, followed by vacuum desiccation. Waste streams from this process, specifically unreacted sulfonic acids and volatile amine vapors, require capture and treatment under chemical hygiene rules. The drive to halve solvent use pushes those in process development to keep improving this route, seeking greener reaction conditions, recyclable solvents, and single-pot synthesis wherever possible.
In applied synthesis, this compound acts as both a nucleophile and electrophile, driven by the imidate moiety’s resonance activity. Alkylation with primary haloalkanes generates substituted amidines, and Michael-type additions across the double bond expand its role in heterocyclic construction. Its sulfonic acid substituent tolerates harsh redox conditions—making it a favorite for multi-step pharmaceutical syntheses where selective reduction or oxidation is required. Copper or palladium-catalyzed couplings use this molecule to build linkers in molecular probes, giving biochemists efficient entry into highly functionalized derivatives. I’ve leveraged its compatibility with both base and acid catalysis to engineer site-selective modifications on peptides, yielding products that maintain activity under physiological salt and pH. The balance of reactivity gives formulation chemists a toolkit for tailoring performance without inviting a rash of side-reactions.
This molecule often goes by alternate designations in catalogs and patents. Some commercial suppliers list it as Ammonium (Z)-N-(2-methyl-1-sulfonylpropan-2-yl)acrylimidate, hinting at its flexible terminologies. In patent filings, names like Prop-2-enimidic acid, 2-methyl-1-sulfopropan-2-yl-, ammonium salt surface regularly. Despite this patchwork, chemical abstracts and procurement systems increasingly reference CAS registry numbers to sidestep confusion, cementing its identity across regulatory boundaries. For those in multi-national research settings, accuracy in documentation avoids regulatory headaches and communication gaps, especially when language barriers introduce transcription errors between runs or facilities.
Handling this compound brings safety front and center. Its hygroscopic nature complicates weighing and dispensing, so gloveboxes or dry-room procedures cut accidental exposure. The dust irritates skin and lungs, causing redness or shortness of breath after just a few minutes without proper PPE. Water runoff from spills can impact aquatic environments—demanding immediate cleanup with absorbent materials and controlled waste management. SDS documentation points out the importance of neutralizing, collecting, and securely disposing of residues, whether in research benches or scale-up reactors. Chemical fume hoods and spill kits aren’t just boxes to tick but essentials that keep research safe and environmentally responsible. Training builds confidence, letting teams work at speed without constantly glancing over their shoulders.
Industries pull value from this compound’s unique mix of properties. In material science labs, researchers use it as a crosslinking agent for specialized resins and adhesives. Catalysis outfits turn to its imidate activity for creating ligands in asymmetric synthesis. Analytical chemists see promise as an ion-pairing agent in liquid chromatography setups, squeezing out sharper separations for complex biochemical samples. I’ve seen pharmaceutical groups integrate it as an intermediate for antihypertensive drug backbones, banking on its ability to tune solubility and metabolic stability. Water purification researchers push its sulfonic acid moiety to attach functional groups that grab onto lead or mercury ions in treatment systems. Even agricultural scientists see the compound as a route to crop protection agents with improved environmental safety over legacy formulations.
R&D waves hit most chemicals in bursts—Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate follows this rhythm. Early work drilled into molecular structure and reactivity; current projects span computational modeling, combinatorial library design, and preclinical studies. Collaboration between academic chemistry departments and industrial consortia keeps driving new derivatives, especially those aimed at niche performance traits—heat resistance, metal chelation, or optimized enzymatic activity. In synthetic biology, research circles try grafting this molecule onto peptides for new biomaterials and enzyme inhibitors. I’ve read studies mapping reaction kinetics and screening conditions, setting benchmarks for new modifications that could leapfrog current limits on synthesis speed or selectivity. Funding from government agencies now supports work on greener production, reflecting wider societal demands for sustainability.
Understanding how chemicals interact with living systems leads directly to safer usage. Studies show that acute oral toxicity falls into the moderate range; skin irritation occurs quickly without barrier protection. Chronic exposure studies continue but thus far suggest lower bioaccumulation compared with some legacy ammonium salts. Wastewater treatment tests flag partial persistence of sulfonate breakdown products, which means end-of-pipe filtration needs attention. I’ve worked with industrial hygienists who monitor area concentrations, confirming best practices align with current workplace limits and safeguard against accidental inhalation. Animal studies keep feeding data into regulatory frameworks, shaping rules around air, water, and soil concentrations for factories and research centers. In vitro tests point to manageable genetic toxicity risks, but surveillance continues to track cumulative effects as usage expands.
Growth in this compound’s adoption may depend as much on environmental stewardship as on synthetic novelty. Green chemistry groups develop catalytic cycles that reuse solvents and cut energy needs—chipping away at the ecological costs of advanced manufacturing. Expansion in life sciences and specialty materials drives demand for newer derivatives that punch above their weight in terms of reactivity and adaptability. Digitized R&D accelerates structure-activity screening, leading to faster launches of custom-tailored analogs in pharma, polymers, and water treatment. Educational programs now need to update training around new best practices, ensuring tomorrow’s chemists walk into labs confident and skilled. As global supply chains see strains from both pandemic aftershocks and regulatory tightening, the resilience and adaptability of this compound’s manufacturing and application base could keep it in the spotlight for years to come. The future of this chemical looks shaped not only by the breakthroughs at the bench, but by the hard and honest work that comes from integrating safety, sustainability, and practical engineering into every step.
Walk into a laboratory, whether academic or industrial, and shelves stand lined with chemicals whose names spark more fear than fascination. Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate sits among this crowd. The name sounds complex, but its story goes deeper than the chemistry textbooks suggest.
This compound serves mostly as a reagent in protein modification and biotechnology research. Scientists lean on it to introduce stable chemical groups onto proteins, especially when studying how proteins work or tracking them in living systems. By providing a specific reactive handle, it lets researchers tag these essential biomolecules without tearing apart their structure, which is no small feat.
In my years around research benches, I’ve seen teams struggle to label proteins cleanly for imaging experiments. Classic reagents often prove too blunt—knocking out the very protein features you’d hope to study. Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate takes a gentler approach. Its unique structure lets it react with select amino acids under mild conditions. As a result, students and postdocs run fewer failed trials, making all those late nights pay off a little quicker.
Protein modification stands right at the core of understanding illness—from cancer and Alzheimer’s to rare metabolic disorders. Precision tools like this chemical let scientists pull apart tangled cellular webs, figure out what’s going wrong, and piece together answers that turn into real therapies. Published studies keep confirming that careful conjugation—the process of adding a tag or functional group to a protein—translates to more reliable data and faster progress.
Alongside protein tagging, some industrial labs test this compound for water-soluble drug development. Its chemical backbone offers ways to alter molecules, improving how drugs move through the body. This sideline doesn’t show up on newsfeeds, yet pharmaceutical development often depends on quietly innovative reagents like this one.
Lab chemicals rarely come without caveats. Handling any ammonium-based compound means paying close attention to safe usage, since some release volatile byproducts when misused. Training remains crucial, especially for young researchers who might treat chemical reagents as just another bottle on a crowded shelf.
I’ve watched chemical stocks pile up in shared spaces—sometimes with missing hazard sheets or empty labels. One path forward includes tighter labeling and regular safety drills, not just for show. Suppliers can also publish safety briefings in plainer language, helping cut down on incidents. For those working in drug discovery, regular testing of experimental residues helps keep projects on track and ensures nothing nasty sneaks through to later stages.
More researchers are choosing eco-friendly reagents as substitutes where possible. For jobs where only Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate fits, labs now test small batches first to spot unforeseen issues. Sharing real-world outcomes in open-access forums can save colleagues headaches. Fewer accidents, more robust results—everyone wins.
The story of this chemical comes down to innovation inside and outside the lab. Reliable reagents let scientists turn ambitious questions into practical discoveries. Keeping safety and open communication at the center doesn’t just protect people—it lays the groundwork for strong, trustworthy science that reaches from the test tube to real patient lives.
Chemicals like ammonium (1Z)-N-(2-methyl-1-sulfopropan-2-yl)prop-2-enimidate don’t usually end up in a standard supply closet for good reason. This compound carries a set of risks tied to both its molecular structure and reactivity. Left unchecked, improper conditions can shorten shelf life or threaten worker safety in a flash. Real experience in the lab tells us that even minor lapses catch up fast, whether the consequences come as a slow loss of potency or an emergency response drill.
Humidity and high temperature speed up chemical breakdown. Ammonium compounds, especially those with imidate groups, pick up moisture from the air and can shift properties or break down completely. A dry, air-conditioned storage room works best. The ideal temperature range runs from 2°C to 8°C, close to standard refrigeration. Warmer storage spaces increase the risk of unwanted reactions, which is more than just a theoretical issue—months of careful work can evaporate with one faulty HVAC cycle.
Desiccators or sealed, moisture-barrier containers stop air and moisture from getting in. Silica beads or similar desiccants offer an extra layer of protection inside storage cabinets. Colleagues often share stories about a single night left open to humidity putting an abrupt end to an entire batch. Prevention here takes far less effort than remediation later.
Light exposure, especially UV light, sets off unwanted side reactions. Storing this compound in amber or opaque glass helps preserve its structure. Storage on a shelf near a window or under harsh lighting slowly wears down the material’s stability, so keeping it in the dark counts as basic good practice.
Cross-contamination may sneak up even faster. The variety of volatile and reactive materials found in research and industrial settings means that segregation matters. Separate this compound from anything acidic, basic, oxidizing, or flammable. Keeping strong chemical odors and vapors away goes hand-in-hand with a locked, well-labeled cabinet. I've heard more than a few stories about mislabeled containers or close-proximity storage cutting projects short before they barely started.
Every organization must follow local chemical safety laws and protocols, whether during storage or transport. Many of these guidelines come from hard lessons learned after incidents. Full tracking of where, how, and for how long a chemical stays in storage always pays off. MSDS—or more recently, SDS—documents line out specific details about hazards, compatibility, and cleanup that shouldn’t be ignored. Logging every access and keeping an up-to-date inventory keeps storage safe and auditable.
Regular training and drills keep everyone sharp. Having clear signage, personal protective equipment at arm’s reach, and updated emergency response plans make the difference between an annoying spill and a major crisis. Investing early in proper shelving, vented cabinets, and environmental monitoring reflects well on everyone’s long-term health and safety outcomes.
Storing this compound isn’t just a technical detail. It reflects the larger culture of care that underpins chemistry and research at every level. Small changes in routine—extra checks, better barriers, smarter location choices—stack up and keep everyone safer in the end.
Chemitry can look like a list of tongue twisters. Still, every complicated name boils down to a set of properties that decide safety. Take ammonium (1Z)-N-(2-methyl-1-sulfopropan-2-yl)prop-2-enimidate—this one rarely comes up outside of specialized labs or certain industrial settings. But for workers and anyone living near facilities where unusual chemicals get used, it's worth asking tough questions about whether a compound is hazardous or toxic.
Companies sometimes bring in lesser-known chemicals as part of cleaner processes or more efficient formulas. That doesn't mean the safety question takes a backseat. Years ago, I wound up with skin irritation from handling a substance everyone around me called “benign,” but no one had checked the latest material safety data sheet. The lesson stuck: don't trust an unproven label or smooth sales pitch.
Most new chemicals head through standard screening to check if they inflame the eyes, irritate the lungs, or cause longer-term problems. For ammonium (1Z)-N-(2-methyl-1-sulfopropan-2-yl)prop-2-enimidate, digging through public databases highlights a gap—a lack of robust toxicology studies or real-world incident reports. Compared to something like ammonia or acrylamide, there isn’t the same body of evidence available.
Hazard clues do pop up, though. This compound’s structure shares features with known irritants. Many ammonium-based molecules cause burning in the throat and nose or damage vegetation if released. Adding a sulfopropan group hints at water solubility, raising the risk of rapid spread if spilled. A related class of imidates also show acute effects if inhaled or swallowed, based on case studies from the European Chemicals Agency and National Institutes of Health databases.
Here’s what’s clear: the lack of a full toxicological report has never meant a green light. In practice, chemical manufacturers sometimes register substances in the United States through the EPA’s new chemicals program, where toxicity and hazard data are often limited to modeling and short-term tests. This system leaves a margin for error—especially for people with prior health problems, chronic respiratory conditions, or compromised immune systems.
There’s no magic shield against the unknown in chemical safety. Industry mistakes from the past—think of Teflon’s PFOA releases or the ongoing fallout from microplastics—show that today’s “safe” label can hide tomorrow’s crisis. The smart move is to treat new compounds with skepticism until the science catches up, particularly where there’s evidence of irritation or persistent environmental risk.
Employers can start by making sure workers see the full safety data and have the right training and personal protective equipment. Local governments benefit from open disclosure about what chemicals are present at nearby sites. Portable sensors for air quality, spill containment kits, and routine surface testing keep a lid on accidental exposure.
Meanwhile, researchers should chase down long-term studies—covering chronic toxicity, cellular changes, and environmental breakdown. Funding for open-access databases goes a long way, too. Transparency equips both workers and communities to push for better protections.
Nobody learns chemical names for fun. Addressing safety demands clear, honest answers—never shortcuts.
Chemistry brings a unique language—one where each part of a molecule’s name tells a story of atomic connections and possible behaviors. Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate stands out with its specific arrangement and naming, and it’s worth exploring that story. I remember the grind of organic chemistry classes in university, searching for meaning in every syllable. A mouthful like this? It holds clues for anyone willing to slow down and puzzle it out.
Anyone who ever spent a semester in a real-life lab knows how crucial accurate naming is. It doesn’t just sound impressive in textbooks or industry documents. It keeps samples from being mixed up, avoids costly mistakes, and makes communication possible across borders.
Here’s what comes across in this compound’s name:
Piecing it together means visualizing a core structure based on prop-2-enimidate, with the N group bearing a 2-methyl-1-sulfopropyl group, pairing as a salt with ammonium. The likely chemical formula shapes into something like C7H16N2O3S for the zwitterionic core, and adding NH4+ from ammonium.
Almost every advancement in pharmaceuticals, agriculture, or materials science hangs on an honest understanding of specific molecular designs like this one. During my time working at a small specialty chemicals startup, precision in naming and structure was directly tied to safety and trust. A mistake could mean lost customers or even accidents in a facility.
Bringing together pieces like the methyl group and sulfonate often turns out to be a balancing act. A methyl may boost hydrophobic character somewhere, while the sulfo group drags it back toward the aqueous phase. Such a combination can influence shelf stability, behavior in solution, and compatibility with other reagents—solutions can’t all be found with trial and error. This is where documented structure and naming matter.
Industry and research both push for greater clarity in chemical descriptions, not just for compliance, but for straightforward collaboration. Clear structure-sharing rests at the core of that. Errors in one step of naming ripple outward—from supplier to supply chain, to end user. If regulations get tighter and greener chemistry becomes the norm, tools and habits for rigorous chemical structure communication grow more valuable.
For chemists, regulators, and manufacturers, the path forward comes with best practices like standardized structure drawings, open-access databases, and regular education around chemical nomenclature. Anyone who’s spent time in the trenches of synthesis knows the frustration of trying to source or use a poorly described ingredient. Keeping strict attention to chemical structure and name remains a basic tool for progress and trust in this field.
Nobody wants to end up with a health scare, a visit from the hazardous materials team, or a ruined experiment. Working with laboratory chemicals like Ammonium (1Z)-N-(2-Methyl-1-Sulfopropan-2-Yl)Prop-2-Enimidate reminded me how important it is to have solid habits. People tend to rely on common sense until they find themselves mopping up a potentially dangerous spill, scrambling for emergency eyewash, or fielding questions from a safety officer. Reading safety data sheets and double-checking storage and labeling make up the foundation. Missing little steps—forgetting to wear goggles or storing incompatible chemicals together—can create big problems down the line.
Gloves, eye protection, and lab coats do more than check boxes on a compliance list. These basics stop skin contact and shield the eyes. Some colleagues grab whatever gloves are handy, but nitrile or neoprene are sturdier choices for protecting against spills and splashes. Cotton lab coats don’t melt or stick like synthetics if a splash reaches the fabric. Chemical splash goggles outperform standard safety glasses by covering the eyes completely. A face shield adds another barrier if there’s any risk of splatter during weighing or transfer.
Ventilation plays a quiet but major role. Fume hoods aren’t decoration in a teaching lab—they’re the lifeline when handling volatile, potentially hazardous substances. I’ve seen researchers skip the hood for “just a quick transfer,” and it often leads to headaches, strong odors, or a round of coughing. It’s better to move all weighing, dissolving, or mixing steps inside a functioning approved hood than breathe in vapors. Keeping the sash at the proper height not only saves the lungs, but it also cuts down on the chance of spills splashing outward.
Organization builds safety from the ground up. Proper labeling prevents mix-ups, especially when abbreviations look similar. Color-coded labels help keep things clear even after a long day. Storing chemicals by hazard class rather than alphabetically helps avoid accidental reactions—acids away from bases, oxidizers apart from organics. Dry, cool storage areas keep decomposition at bay.
Small spills get ignored more than they should. I remember a researcher wiping a tiny drop up with a bare hand, then returning to the keyboard, which put everyone else at risk. Spill kits belong close to every workstation, with absorbent pads, gloves, and neutralizers. Reporting even a small incident gives the safety team a chance to track recurring issues and keeps records clear.
People often leave safety training in the background, but hands-on demonstrations have much more sticking power. Practicing emergency routines—like heading to the safety shower with closed eyes—shows the real difficulty of using safety equipment under stress. Creating a culture where colleagues remind each other about PPE, or where a new team member gets a walkthrough before starting, keeps everybody alert. Looking out for each other brings a sense of responsibility that goes past just following rules.
Building strong habits makes labs safer for everyone. Pairing personal protection with reliable ventilation offers a double barrier. Accurate labels and good organization help avoid mistakes. Reporting and cleaning up even small spills stop problems from growing. Ongoing, experience-based training weaves safety into the everyday work routine, and that steady commitment stands as the best defense against both accidents and lost research.
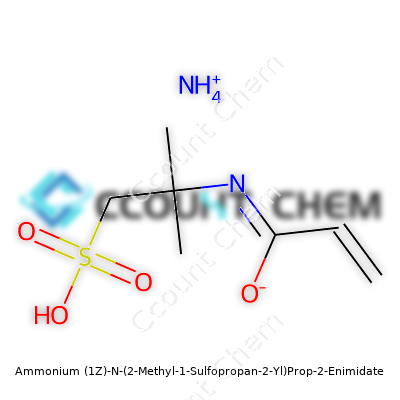
| Names | |
| Preferred IUPAC name | Ammonium (Z)-N-(2-methyl-1-sulfopropan-2-yl)prop-2-enimidate |
| Other names |
AMSP Ammonium (1Z)-N-(2-methyl-1-sulfopropan-2-yl)prop-2-enimidate 2-methyl-1-sulfinopropane-2-imidate ammonium AMSP (abbreviation) |
| Pronunciation | /əˈmoʊniəm wʌn zɛd ɛn tuː ˈmɛθəl wʌn ˈsʌlfoʊˌproʊpən tuː aɪl prɒp tuː ɛn ɪˈmɪdeɪt/ |
| Identifiers | |
| CAS Number | 1777356-45-4 |
| 3D model (JSmol) | `JSmolModel("C7H14N2O3S")` |
| Beilstein Reference | 2147003 |
| ChEBI | CHEBI:147485 |
| ChEMBL | CHEMBL2105937 |
| ChemSpider | 12682716 |
| DrugBank | DB11262 |
| ECHA InfoCard | 03-2119943722-46-0000 |
| EC Number | 260-709-4 |
| Gmelin Reference | Gmelin Reference: 1048336 |
| KEGG | C19749 |
| MeSH | D000647 |
| PubChem CID | 25199674 |
| RTECS number | VN8290000 |
| UNII | 89J2V17L7A |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C7H15N2O3S |
| Molar mass | 241.31 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.17 g/cm3 |
| Solubility in water | soluble |
| log P | -2.0 |
| Acidity (pKa) | pKa = 10.75 |
| Basicity (pKb) | pKb = 4.3 |
| Refractive index (nD) | 1.431 |
| Dipole moment | 4.3471 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 316.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -526.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1054.7 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: > 100°C |
| LD50 (median dose) | LD50 (median dose): Rat oral >2000 mg/kg |
| NIOSH | NIOSH: BQ9028000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 0.02 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Sodium 2-methyl-2-propene-1-sulfonate Ammonium methacrylate Methacryloyl imidate Ammonium (1Z)-N-prop-2-enimidate 2-Methyl-2-propenamide |