Aminosulfonic acid, also known as sulfamic acid, started seeing chemical interest in the early 19th century as researchers progressively mapped out the behavior and synthesis of new compounds. Chemists like Justus von Liebig and other pioneers struggled with isolation and analysis at a time when laboratory tools looked nothing like today’s precision glassware. The compound really started finding traction across European laboratories in the wave of industrialization that followed, as benefits like non-volatility and unique reactivity made it stand apart from mineral acids. By the mid-1900s, improvements in purification and bulk synthesis drove down costs, making it attractive for mass production and inclusion in products ranging from household to heavy industry.
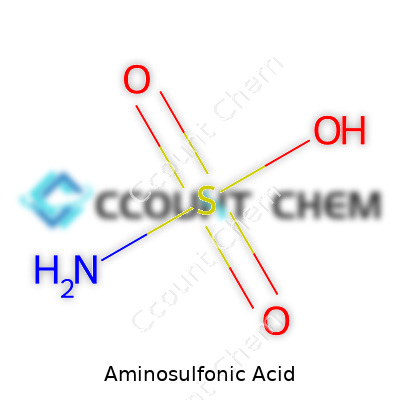
A white, odorless crystalline powder, aminosulfonic acid steps into countless industries with surprising versatility. Its solid form and high melting point benefit shipping and storage, unlike liquid acids prone to leaks or evaporation. In practice, chemical companies deliver it in bags or drums, optimally stored away from moisture. Customers often look for it in cleaning agents, pH adjusters, and descalers, as it stays easy to use and mixes well in formulations. Its straightforward structure (NH2SO3H) combines an amine group with a sulfonic acid, pairing two reactive ends that always catch chemists’ attention.
Aminosulfonic acid appears mostly as colorless crystals or a fine powder, displaying strong solubility in water but far less in solvents like ethanol. The melting point lands around 205°C, showing impressive heat stability for such an accessible acid. Unlike volatile mineral acids, it keeps to itself in powder form, giving off no toxic fumes at room temperature. Its pKa (sulfonic group) rests near 1, producing acidity useful for reactions and applications requiring non-volatile, manageable acid behavior. Solutions of aminosulfonic acid stay clear and attack mineral scale with vigor but show limited action against metals, sparing pipes and surfaces that stronger acids would chew up.
Manufacturers rely on clear benchmarks to grade aminosulfonic acid. Purity typically measures above 99%, flagged on labels with only tolerances for moisture and insoluble residues. Labels reflect batch lot, production date, and standardized hazard ratings based on GHS or OSHA, indicating corrosion dangers. Guaranteeing stable moisture content supports predictable reactivity, particularly in industrial cleaning or food-processing situations demanding repeatable performance. Regulatory authorities in regions like the EU or the U.S. monitor labeling closely, pressing manufacturers to communicate the safety and composition details without ambiguity for downstream users, whether chemical plant managers or janitorial staff.
The classic route starts with urea and fuming sulfuric acid as feedstocks, exploiting urea’s amine and sulfuric acid’s sulfonation power to yield sulfamic acid and carbon dioxide. Technicians meter reactants slowly, riding a tightrope between heat removal and careful agitation, since exothermic spikes risk side-reactions that cut yield and spoil purity. This synthesis works at large scale too, giving the chemical industry leverage for millions of tons each year. Filtration and crystallization polish up the product, drawing from decades-old engineering lessons fine-tuned for mass efficiency.
Aminosulfonic acid stands eager for reaction, giving up its proton with ease to neutralizers like ammonia, potash, or lime. These salt forms, like ammonium or potassium sulfamate, open other industrial doors. Strong oxidizers, such as nitric acid, force decomposition, producing nitrogen oxides and sulfur oxides, which limits mixing choices. It resists hydrolysis under mild conditions, extending shelf life in wet blends. Scientists tinker with substitutions on the amine or sulfonic groups, engineering derivatives for targeted roles—the kinds that block enzyme activity in pesticides or fine-tune solubility in specialty cleaners. Even in labs, sulfamic acid has a place for nitrite decontamination, showing how classic molecules still pull their weight in modern settings.
Through history and across continents, aminosulfonic acid travels with a handful of aliases. The chemical trade most often calls it sulfamic acid, but synonyms like amidosulfonic acid or amidosulfuric acid appear in technical documents. Manufacturers assign trade names that blend chemistry with branding, such as Sulfamat or Aminosulfuric, to anchor recognition and trust. Publications and safety databases sometimes tag numbers like CAS 5329-14-6, cementing a link between nomenclature and regulatory attention. These diverse names shadow the same utility, uniting cleaning, water treatment, and specialty industries under familiar chemistry.
Aminosulfonic acid’s low volatility limits inhalation risk under normal use, but concentrated solutions still corrode living tissue, especially the eyes and skin. Handlers rely on gloves, goggles, and, in higher concentrations, face shields and ventilation to control exposure. Waste solutions, neutralized before sewer discharge, keep municipal systems safe and legal. Bulk sites that store tons of sulfamic acid keep emergency protocols up to date, drawing lessons from the more dangerous acids to avoid complacency. International standards like ISO and local workplace guidelines drive training programs, aiming for zero-incident operation and awareness around handling and spills.
This acid quietly powers a range of household and industrial jobs. Households depend on it in scale removers, tackling lime and rust stains in kettles or pipes without metal corrosion. Power plants and food factories value its balance—strong enough for performance, gentle enough not to chew apart core equipment. Paper manufacturers, dye houses, and even pharmaceutical companies call on its reactivity for purification, synthesis, and as a pivotal intermediate in reactions. The ability to neutralize sodium nitrite under controlled conditions keeps water safe and makes it a staple for public utilities. Lawns and gardens see it in herbicide production, extending chemical agriculture’s reach. Even fire retardants sometimes draw from ammonium sulfamate, made directly from the parent acid.
Chemists continue to push the limits of aminosulfonic acid, optimizing its use in catalysis and as a green substitute for older, dirtier mineral acids. Researchers explore combining it in resin or polymer preparations to engineer resistance against fouling or scale. Academic labs experiment with its role in developing nonlinear optical materials for lasers and sensors, chasing new pathways in electronics and medical diagnostics. Collaboration between chemical manufacturers and universities steadily broadens its portfolio, from extending food-grade certification uses to trialing bioremediation methods, where sulfamic acid neutralizes threats in soil or water after environmental spills.
Aminosulfonic acid tends to show low toxicity when ingested in small amounts, but safety studies consistently warn against complacency. Prolonged skin or eye exposure burns tissue, as expected with strong acids. Respiratory studies confirm little risk from dust at low concentrations but raise flags in work settings with heavy powder handling. Chronic toxicity data remain scarce, with animal studies suggesting low systemic harm unless doses run high, but regulatory agencies push for high clarity—hazards marked clearly, exposure limits grounded in modern research, and routine updates as new findings emerge. Recent work addresses breakdown products, with environmental tests suggesting limited persistence but highlighting a need to track release into waterways or soil over time.
Demand for aminosulfonic acid looks set to keep climbing as societies call for greener chemistry and products that balance power with safety. Manufacturers eye upgrades to cheaper, less energy-intensive production processes, hoping to trim the carbon footprint of chemical supply chains. In green cleaning and precision electronics, gentle but targeted acids like this one stand a solid chance against tougher competitors that fall short on environmental metrics. Chemists experiment with biodegradable derivatives, hoping for next-generation cleaning agents or scale removers that vanish safely after use. Regulatory scrutiny will likely tighten, lending an edge to producers that document safety, traceability, and environmental impact from factory gate to end-user. This acid’s modest profile stands little chance of disappearing while industries and homes keep asking for reliable, cost-effective solutions that ride the fine line between performance and protection.
Most people come across aminosulfonic acid without even knowing it. Plenty of folks use descaling powders for kettles or coffee machines. That powder working away on your limescale often contains this ingredient. Smart chemists value it for one big reason: it deals with hard water deposits—and fast. The acid eats away at the minerals causing white, crusty build-up on anything from showerheads to industrial boilers.
Big factories also rely on aminosulfonic acid to keep their equipment running. In my manufacturing days, keeping pipes and heaters clear saved us headaches and money. Gunk and mineral sludge make machines work harder, chew through energy, and push maintenance costs through the roof. Routine cleaning with this acid made sure we didn’t replace expensive parts more than needed.
Anyone who’s managed a swimming pool knows cloudy water spells trouble. Aminosulfonic acid, often sold as cyanuric acid or chlorine stabilizer, helps keep pool water balanced. By controlling how fast chlorine breaks down in sunlight, it keeps pools safe from bacteria and algae. For folks running a public pool or a local gym, this means safer water with less fuss over daily treatments.
In the medical field, aminosulfonic acid shows up in gastroenterology. Doctors sometimes use it as a supplement for people with certain metabolic issues. It helps break down food and supports certain body functions, though you wouldn’t start taking it without clear advice from a medical professional. Reliable studies from groups like the National Institutes of Health back up its use in specific therapies, but everyday folks don’t take it like a multivitamin.
The chemistry behind aminosulfonic acid is solid and well documented. Research published by the American Chemical Society shows it works as a cleaning agent because it’s both strong and predictable. Its use leaves less risk of dangerous fumes compared to more aggressive acids like hydrochloric or sulfuric acid, especially in closed environments.
No one should ignore safety just because something is common. Aminosulfonic acid, if handled wrong, burns skin and eyes and can cause lung issues if inhaled. That’s one reason real training matters—labels on containers don’t tell the whole story. I always wore gloves and goggles and kept the place ventilated. Taking these steps cut down on emergencies and kept my team safe.
The chemical runs off into waterways when big facilities clean with it repeatedly. Regulators and watchdog groups notice. Clear runoff standards, coupled with water recycling where possible, can cut down environmental impact. I’ve worked with local governments setting up wastewater treatment with neutralization steps, so the acid doesn’t end up in the river downstream.
Demand for stronger, faster cleaning will stick around, and aminosulfonic acid fits the bill. The best approach: use it where it outperforms safer, gentler options. Transparent communication through safety sheets, and staff trained in real risks, matter just as much as technical breakthroughs. A good tool used the right way can solve more problems than it creates. That’s worth remembering, both in a busy factory or around the house.
Aminosulfonic acid, also called taurine, often pops up in bold letters on the back of energy drink cans and some nutrition supplements. Despite being a common ingredient, I’ve noticed folks rarely talk about what it actually is or if it’s really safe. The name might sound intimidating, but it’s something the body makes on its own. In fact, I first heard about taurine in the context of cat food—turns out, cats can’t produce it, and need it from their diet, or they run into real health problems. Humans are luckier; our bodies produce enough under normal circumstances, though some people wonder if getting extra helps.
Researchers have dug into taurine for decades, since it plays a big role in heart health, eye function, and even brain chemistry. People get it from meats and fish, not from plants, so vegans and vegetarians might not have as much. The U.S. Food and Drug Administration lists taurine as “generally recognized as safe” for consumption in normal amounts you’d get from food or the occasional energy drink. Europe’s Food Safety Authority looked at some massive doses—up to 6 grams a day—and didn't turn up any serious problems in healthy adults over a short term.
Energy drinks sometimes heap on taurine, delivering up to 1000 milligrams in one go, mixed up with caffeine and sugar. Drinking that stuff fast can leave some people jittery, lightheaded, or just off. I remember picking up a flashy can during a late-night study session in college and feeling my heart pounding—not just from the caffeine, but maybe from other ingredients at play. Doctors warn that too much of any one thing, especially alongside heavy doses of caffeine, could tip someone over. Taurine alone rarely causes trouble; it’s mixing it with stimulants or using it as a quick fix for fatigue that sets off alarms.
Out on forums, plenty of people talk about adding taurine powders or pills to their diet, hoping for a cognitive edge or better athletic performance. The science doesn’t really back up wild performance claims, though. Athletes and gym-goers sometimes report fewer cramps or feeling more steady, but these results tend to be subtle or short-lived. Folks with kidney issues or those who take certain medications might need to avoid extra taurine. Most doctors I’ve talked to recommend getting nutrients from a balanced diet, not chasing miracles in a bottle or a can.
Taurine shows how even a naturally occurring compound can raise safety concerns if overused or paired with other substances. Just because something shows up in energy drinks or supplements, that doesn’t mean more is better. Deciding what’s “safe” comes down to dose, how it interacts with other things, and most importantly, the overall health of the person thinking about using it. Looking up reviews from trusted health agencies, asking healthcare providers, and sticking to amounts found in foods usually serves as a smart path. Supplements can play a role for some, but they shouldn’t replace good sense or a decent meal.
Aminosulfonic acid, better known as taurine in some corners of the supplement market, pops up everywhere from energy drinks to cleaning products. After reading too many labels and asking too many pharmacists about ingredients, I learned the importance of looking past flashy claims and checking for possible side effects, no matter how common a name sounds. To speak honestly about what people might face after ingesting or handling aminosulfonic acid, it makes sense to rely on research and the real stories floating around doctors’ offices and households.
Some folks don’t even realize that those mild cramps, upset stomach, or that strange urge to always look for a bathroom can come from products listing aminosulfonic acid. Bloating and diarrhea also catch people off guard. Scientific reports, like one published by the American Journal of Clinical Nutrition, show that improper dosing or a sudden increase in intake can upset the digestive tract. Those who start taking supplements with aminosulfonic acid, or use industrial cleaners that splash onto their skin, report nausea and occasional vomiting. These symptoms usually fade after people adjust intake, but that doesn’t make the initial discomfort easier to brush off.
Most people probably associate aminosulfonic acid with energy drinks, but factory workers and lab staff bump up against it in raw form. Direct skin exposure leads to redness, itching, or even rashes. My uncle, who worked in a detergent plant, talked about spots and irritation that showed up after a few hours without gloves. Asthma flares and breathing troubles also come up with dust or fumes, especially in places without proper protective gear. The Occupational Safety and Health Administration lists aminosulfonic acid as a substance worth handling carefully for this reason: Excess exposure without masks or fans makes it tough to breathe easily.
Taurine supplements sound harmless, maybe because they’re described as “naturally occurring.” Yet, taking in high amounts throws off more than just the gut. Research shared by The Journal of Pharmacology uncovers changes in electrolyte balance, which plays with everything from heart rhythm to muscle cramps. That same imbalance can lead to lightheadedness, fatigue, or confusion in some people who were otherwise healthy before chugging multiple servings a day. It’s not just about “feeling a bit off.” People with kidney or liver troubles should avoid products containing aminosulfonic acid without full sign-off from their care provider, since their bodies may not clear it out as quickly as expected.
People want results. High energy, clean homes, or extra strength in the gym sound great, and companies know this. Reading labels and talking to healthcare professionals goes a lot further than trusting online posts or assumptions. Manufacturers can help by switching to safer packaging and printing real warnings about side effects so nobody gets a nasty surprise. Reporting strange symptoms and sharing real stories, whether it’s after a workplace exposure or a supplement binge, helps researchers and doctors piece together a bigger picture. Nothing beats respect for the body’s signals. If something feels wrong, pausing and getting help keeps small problems from turning into bigger ones.
Aminosulfonic acid finds its way into daily factory life—cleaners rely on it, water treatment experts turn to it, farmers may recognize it from fertilizer labels. Whether you handle a few bottles in a lab or manage pallets in a warehouse, how you store it shapes everything from product quality to workplace safety.
People know aminosulfonic acid as sulfamic acid. It resembles crystalline powder, dissolves in water, and reacts with oxidizers like bleach. Inhaling dust can irritate lungs, while direct skin or eye contact feels unpleasant, sometimes hazardous. Over the years, I’ve seen accidents start with careless storage, even when workers had the right safety gear. Someone grabs a damp scoop, or leaves a lid loose, and suddenly you’re facing a cleanup instead of your usual business.
The answer isn't only in high-tech storage rooms or fancy labeling. Small steps carry huge weight. I always store this acid in sealed containers. Any moisture sneaking in causes clumping, ruins batch consistency, or triggers early chemical reactions—no one wants that, especially with cost and quality on the table. Plastic drums or thick polyethylene bags work well. Metal can rust, especially under humid conditions, and loose cardboard boxes give way too easily.
Temperature changes bring their own problems. Stacking bags by a window or under direct sun cooks the acid, while cold, damp corners cause condensation. Steady room temperature, out of sunlight, beats both extremes. I learned long ago that climate control, even if simple, stops headaches down the line.
Workers sometimes see a white crystalline material and forget the hazards. I remind people to use gloves, safety glasses, and masks. No one wants a trip to the clinic just because they rushed a job or ignored basic PPE. Training doesn’t sit as a one-time event. It’s about reminders, checking that labels stay readable, keeping emergency wash stations close to where the work happens.
In the unfortunate event of a spill, keeping clean-up gear close by cuts down reaction time. Absorbent materials, brooms, dustpans—not luxury, just simple preparedness. Companies shouldn’t store unrelated chemicals side by side with aminosulfonic acid, especially oxidizers or strong bases. Dangerous reactions—sometimes explosions—have happened in places that mixed their storage out of convenience.
Certifications, like ISO, don’t solve every problem. Real solutions come from clear rules that everyone actually follows. For new staff, hands-on demonstrations shed more light than a stack of paperwork. I like keeping a storage checklist taped where staff can see it: dry? sealed? labeled? Not complicated, but it helps every single shift.
Some companies let a safety manager walk the floor monthly, noting clutter or leaky containers. This isn’t red tape. This is insurance for the business. Investing a little time each week guarantees fewer surprises, longer shelf life, and a safer workday for everyone involved.
So, whether you're working in a high-throughput plant or a local lab, spending time to tidy up and store this acid well isn’t only following regulations—it's common sense grown from real experience and respect for both people and product.
Aminosulfonic acid, which you might hear called taurine, serves widely in food supplements and some energy drinks. Beyond the sports and gym crowd, doctors sometimes consider it for specific medical conditions where the body’s amino balance falls out of order. This stuff isn’t just for weightlifters or those drinking neon-colored beverages—it’s present in all of us, mostly produced in the liver.
Dosing any supplement without clear evidence can get risky. With aminosulfonic acid, safe dosage often depends on why you’re taking it. For regular, healthy adults, studies have used amounts from 500 milligrams up to 3,000 milligrams daily without showing harm. The World Health Organization reviewed the available science on taurine about twenty years ago and judged up to 3,000 mg per day likely poses little risk for most healthy folks.
That said, scientists haven’t figured out an exact dose that fits everyone. The research points to individual differences in needs. People with certain genetic conditions or long-term illnesses might process amino acids differently, and children metabolize supplements in their own way. Jumping on a standard number can ignore the way our bodies respond to nutrients and supplements over time. No matter what’s trending in wellness circles, it makes sense to actually talk to a doctor who can spot signs of trouble or overdose before they happen.
A lot of people turn to aminosulfonic acid for claims about sharper focus, better sports performance, or a healthier heart. The evidence keeps evolving, with some studies showing promise for muscle cramps or improving heart function, especially in people fighting congestive heart problems. In my own family, doctors sometimes mentioned taurine as a supportive element for rare metabolic issues, recommending it in carefully measured doses tied to blood work. It wasn’t a case of “more is better”—more often, we paced the dose and checked lab tests along the way.
Energy drinks, on the other hand, pack significant doses. Some products combine taurine with caffeine and other ingredients without much clarity on long-term safety. In reality, the blend of sugars, stimulants, and amino acids complicates the picture. The single-dose recommendation for a supplement doesn’t always match what people get from a can of an energy-boosting beverage.
Going overboard with aminosulfonic acid can strain organs, especially in those with kidney disease. Nausea, headaches, or trouble sleeping pop up for sensitive people even at modest intake. When new supplements hit the market, nobody wins if the label hides how much is safe or blurs the line between nutrition and self-experimentation. Transparency serves everyone better: companies listing exact dosages and providing clear safety warnings build trust and keep folks safe.
Doctors and nutritionists both play a role in guiding real-world dosing. More than once, I’ve watched a family friend try a supplement based on a gym peer’s advice—later learning a quick call to a pharmacist or physician would have spared days of discomfort. Sticking to the research, checking with health professionals, and slowing down on megadoses beats chasing quick gains.
Aminosulfonic acid doses fit best when tied to individual needs, usually falling in the 500 to 3,000 mg daily range for healthy adults. Specific medical situations may call for different amounts under professional supervision. Open information, honest medical guidance, and real science all matter far more than trends when it comes to safe supplement use.
| Names | |
| Preferred IUPAC name | sulfamic acid |
| Other names |
Sulfonic acid, amino- Aminosulfonic acid Sulfoamino acid Aminosulfonate Sulfoaminate H2NSO3H Sulfamic acid |
| Pronunciation | /əˌmiːnoʊˈsʌlfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 51-21-8 |
| Beilstein Reference | 1713883 |
| ChEBI | CHEBI:16027 |
| ChEMBL | CHEMBL1276 |
| ChemSpider | 546 |
| DrugBank | DB01942 |
| ECHA InfoCard | 03c3b7a7-3170-4416-b52e-c7c061bfd3ec |
| EC Number | 205-488-3 |
| Gmelin Reference | 63590 |
| KEGG | C00191 |
| MeSH | D002442 |
| PubChem CID | 6112 |
| RTECS number | B03332100 |
| UNII | F0LCU2MZN3 |
| UN number | UN2967 |
| CompTox Dashboard (EPA) | DTXSID1020389 |
| Properties | |
| Chemical formula | NH2SO3H |
| Molar mass | 97.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | soluble |
| log P | -4.34 |
| Vapor pressure | 0.08 hPa (20 °C) |
| Acidity (pKa) | 1.0 |
| Basicity (pKb) | 1.00 |
| Magnetic susceptibility (χ) | -6.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | 1.64 mPa·s (20 °C) |
| Dipole moment | 3.37 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 81.3 J⁄(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -530.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -565.8 kJ/mol |
| Pharmacology | |
| ATC code | B05XA06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye damage, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P301+P330+P331, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | 130 °C |
| Autoignition temperature | ~440 °C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 3160 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 3160 mg/kg |
| NIOSH | 7703 |
| PEL (Permissible) | PEL = Not established |
| REL (Recommended) | 50-100 mg/L |
| IDLH (Immediate danger) | 1000 mg/m3 |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Sulfamic acid Sulfanilic acid |