Tracing the roots of amidosulfonic acid, more often called sulfamic acid, starts in the late 1800s. Early chemists experimented with modifying urea and sulfur trioxide, chasing after ways to tame harsh acids for practical tasks. Some saw promise in its mild, stable form—easier to handle than sulfuric acid, yet strong enough to clean and remove stains that other compounds could only smudge around. Over the last century, innovation and tightened manufacturing processes boosted amidosulfonic acid from a laboratory curiosity to a backbone of industrial descaling and water treatment. Discoveries along the way included methods to purify it for sensitive uses, plus safer production routes that fit with tighter workplace safety rules.
Found as white, crystalline granules, amidosulfonic acid holds a unique spot on the shelf for cleaners, water treatment specialists, and researchers. Once folks realized its calcium and oxide-dissolving power and the fact it didn’t emit choking fumes, commercial demand spiked. The compound keeps a stable shelf life under dry, well-sealed conditions. Manufacturers sell it in bags, drums, or high-purity bottles, each labeled with batch details, manufacturing date, purity percentage, and clear handling recommendations. Sometimes it arrives under names such as sulfamic acid, amidosulfonic acid, or as its salt derivatives in formulary handbooks and shipping logs.
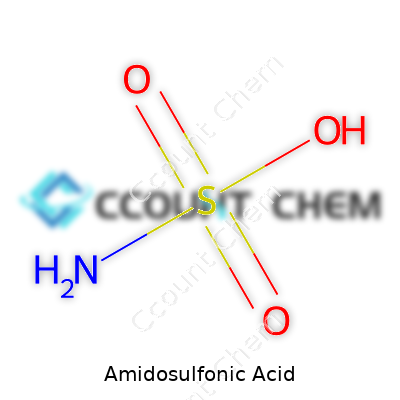
Amidosulfonic acid sits at the intersection of acids and amides. Its chemical formula, H3NSO3, shows a close kinship with sulfuric acid and urea. What makes it stand out is its nonvolatile nature and water solubility. Add it to water and you'll watch the granules dissolve quietly, giving off little heat—nothing like the splatter you get from traditional mineral acids. It melts around 205°C and breaks down at higher temperatures, so lab techs know to keep heat below that mark. On the pH scale, a fresh solution lands squarely in the acidic range, making it an effective agent for breaking down limescale or rust. Most impurities or water hardness stand little chance against its action, yet it stays gentle on metals compared to some harsher alternatives.
Every bulk drum or laboratory vial arrives with technical sheets noting purity, usually above 99.5% for reagent-grade batches. Impurities such as heavy metals, chloride, and iron stay well below one part per thousand. Safety tags repeat hazard statements and emergency cleanup steps. Labels must spell out the product’s chemical name, adorn the container with hazard pictograms, and confirm batch traceability. This level of detail comes straight from both international transport rules and regional chemical safety frameworks—something folks in the industry grew to demand after several high-profile mixing mishaps in the 1970s and 1980s prompted tighter standards. Quality control teams use titration or spectroscopic checks to guarantee every bag meets the posted figures.
Making the acid draws on a reaction between urea and sulfur trioxide, often under anhydrous conditions to sidestep dangerous splattering. Operators run the process in sealed reactors fitted with local ventilation, careful to balance each ingredient by weight. By keeping water out, chemists dodge runaway exotherms and get a clean crystalline product without extra purification steps. This process, once tricky thanks to the hygroscopic nature of urea and the reactivity of sulfur trioxide, came under tighter control in the post-war years. Engineers now use continuous monitoring and temperature control to ensure reproducibility, helping the acid remain cost-effective for both niche and large-scale applications.
Lab workers often reach for amidosulfonic acid when they need a reliable acid that won’t throw off unwanted side reactions. Its main chemistry involves releasing hydrogen ions in water and acting as a safe stand-in for hydrochloric or sulfuric acid, especially if vapor pressure or volatility become sticking points. Mix it with nitrites and it turns into nitrogen, while strong heating can split the molecule into sulfur trioxide, water, and ammonia—fumes you want nowhere near your face mask. Industry has found ways to tweak its basic form, making potassium or sodium sulfamate salts for selective herbicides, or as mild acidifiers in metal finishing baths. Tweaks for scale inhibitors or dye intermediates pop up in patent filings from Japan, Germany, and the US, each building on the same core reaction profile.
The world of chemistry rarely settles on just one name. Official texts recognize “sulfamic acid” in English, “acide sulfamique” in French, and “acido solfammico” in Italian catalogs. In industry, you’ll see codes like UN 2967 for shipping, or commodity numbers embedded in customs paperwork. Specialized supply houses use trade names, sometimes combining the technical term with their own branding. Research papers list it as both H3NSO3 and sulfamic acid, depending on the writer’s origins. It's always worth walking through the synonyms before ordering, as a mislabeled drum can cause complications, especially during cross-border work or regulatory inspections.
Anyone working with amidosulfonic acid in bulk learns to respect its corrosive punch, but also appreciates the generous safety window compared to sulfuric or hydrochloric acid. Contact with eyes or mucous membranes irritates right away, so gloves, goggles, and lab coats always come out before any transfer. Markets in North America, Europe, and Asia follow specific occupational exposure limits and suggest strong local ventilation in mixing or bottling rooms. Cleanup of spills calls for neutralization with sodium bicarbonate or an alkaline solution, never water directly on powder to avoid heat or splatter. Training programs hammer these points home, and regular safety audits catch any lapses. New research keeps updating standards, pushing toward less packaging waste and safer, tamper-resistant closures for both consumer and industrial use.
Descaling stands out as the top use for amidosulfonic acid—think heat exchangers, coffee machines, and municipal water systems clogged with stubborn scale or rust. Beyond cleaning, it finds a home in dye manufacturing, where it helps couple colors to fabrics with the right acidity. Metal finishing plants rely on it to prep surfaces before plating, usually instead of mineral acids that would attack both staff and base materials. Water treatment crews drip-feed precise doses to keep boilers or cooling loops clean without corroding pumps and valves. Specialty food producers look at it for cleaning dairy or brewing lines, appreciating that residues wash away without chlorine taste or aftertaste. As a fertilizer processor, I watched hundreds of tons help maintain lines with minimal maintenance and downtime, proving its utility across agriculture, textiles, energy, and research.
Researchers push the frontiers with amidosulfonic acid, tracing out new uses in catalytic methods, battery chemistries, and environmentally friendly herbicides. Universities and corporate labs look for ways to tweak its core structure for more targeted action in industrial cleaning and as intermediates for pharmaceutical synthesis. Analytical chemists see promise in its precise reactivity for titration studies and new forms of solid storage for acids. Ongoing efforts push for greener production, aiming for bio-based synthesis or milder processing solvents. Emerging techniques use computer modeling and high-throughput screening to sort through modifications that improve performance or decrease toxicity, always watching regulatory shifts and market feedback.
Toxicologists put amidosulfonic acid through rigorous testing, scrutinizing both acute toxicity and long-term effects. Oral LD50 values in rats usually fall in the high hundreds to low thousands of milligrams per kilogram, classifying it as moderately hazardous but not as dangerous as many industrial acids. Skin contact causes irritation, so regulatory bodies stress personal protective equipment and proper ventilation. In water, the acid breaks down rather quickly, and studies find it has limited mobility in soil—signs that spillages can be managed with proper response. Chronic inhalation or exposure in enclosed environments shows increased risk for respiratory irritation, underscoring the need for robust workplace controls and clear material safety data sheets. Monitoring of effluent streams helps reduce any environmental impact, as regulations limit discharge and require neutralization at water treatment plants.
Looking forward, amidosulfonic acid stands poised for greater roles in both clean manufacturing and next-generation materials synthesis. Demand ticks up as industries chase alternatives to more hazardous acids, especially as stricter environmental rules squeeze old cleaning and finishing methods. Researchers forecast expanded use in energy storage, textile dyeing using less water, and biotech fermentation lines where traditional acids prove incompatible. Greener synthesis methods, lower-toxicity salt forms, and embedded sensors for real-time acid monitoring could shape its path in the coming decades. In my work, the movement toward sustainable chemistry always circles back to safe, versatile compounds like amidosulfonic acid—less glamorous than flashy catalysts or rare metals, but no less essential to making progress both possible and responsible.
Walk into a chemical supply room, and you'll likely spot amidosulfonic acid on the shelf. Most folks working outside chemistry circles know it as sulfamic acid. It never gets the spotlight, but this white, odorless powder holds surprising value in all sorts of everyday industries.
Factories depend on keeping pipes and machinery clean. Scale and mineral buildup in boilers cut down efficiency and gobble up energy. Drop sulfamic acid into the mix, and limescale vanishes like magic. No ironic twist or fancy background needed; it just works. Crews appreciate how it tackles rust and deposits in water treatment plants and commercial kitchens. An old friend of mine who ran a pool maintenance business swore by it for stubborn stains and clogged filters. He saved on repairs for years just by working smarter, not harder, thanks to this stuff.
The food world can’t mess around with dirty surfaces or hidden residues. Sulfamic acid serves as an unsung hero in factories. Tanks, valves, and other equipment pick up protein buildup and calcium scale. Instead of risking contamination or damaging expensive gear, workers use this acid for scheduled deep cleaning. Because it leaves no weird aftertaste or toxic trace, it makes sense for spots where clean means safe.
Paper mills churn out thousands of tons each day. They don’t want anything gumming up the works. Sulfamic acid helps bleach pulp without harsh byproducts. Factories favor it for the simple reason it breaks down easily, so the process doesn’t clog streams or foul up downstream equipment. Nobody wants paper that smells off or leaves hidden residue, and this acid helps meet those tight standards.
Swing by the cleaning aisle at any grocery store, and you’ve probably noticed products for de-scaling kettles, coffee machines, or bathroom limescale. Look closer at the label: sulfamic acid pops up often as the main ingredient. Its power comes with a practical advantage—it won’t eat away at metal or skin like stronger acids. Safety in the home matters, and that's where this chemical meets demand with less risk and lots of cleaning punch.
Textile makers rely on sharp and lasting colors. Water with too much mineral content messes up dyes and throws off batches. Adding sulfamic acid to the rinse fixes this problem easily. Colors stay bright, and there’s no crust forming in dye vats. Workers see better results without extra downtime, meaning less waste and tighter production.
Any chemical has downsides if handled carelessly. Sulfamic acid ranks lower on hazard scales compared to many strong acids, but it needs respect. Companies train staff to use gloves and eye protection, and they track exposure. Responsible storage and disposal keeps it out of landfills and water supplies. Researchers and regulators keep an eye on workplace levels and environmental impact to catch problems before they spread.
We sometimes overlook what's hiding behind clean drinking water, scale-free boilers, or smooth paper. Reliable products like amidosulfonic acid don't grab headlines, but they support food safety, efficient manufacturing, and simple comforts in daily life. With the right balance of use and caution, it helps industries keep running while protecting workers and the environment—for everyone’s benefit.
Amidosulfonic acid, often called sulfamic acid, shows up in a lot of cleaning products and descalers. In many households, it sits in toilet bowl cleaners or scale removers for kettles and coffee machines. Chemists reach for this stuff because it dissolves limescale without the rough smell or fumes of stronger acids. On an industrial level, maintenance teams clear out mineral build-up using it.
People sometimes see “acid” and feel concern—fair enough. Acid burns, right? With amidosulfonic acid, the story feels a little nuanced. Solid flakes or powder won’t eat through your skin in seconds, but ignoring safety means risking irritation, especially if it gets in your eyes, nose, or mouth.
One thing I’ve noticed in working around various chemicals is how far a little respect and awareness go. If you breathe in dust, you could cough or irritate your breathing passages. Touching it routinely without gloves dries out your skin and may cause redness or broken skin. The biggest concern, though, comes if powder splashes in your eyes—this can feel intensely painful and could cause real damage if not flushed quickly with water.
Everyday products dilute the acid pretty well, so risk drops off there. Descaling a kettle or scrubbing the bathroom, you’re usually working with weak solutions, so the harm is lower, but not zero. People sometimes mix household chemicals, maybe to “make something stronger.” This mistake can create toxic gases or increase the risk of burns.
European Chemicals Agency (ECHA) research confirms that amidosulfonic acid irritates skin and eyes. Strong concentrations burn eyes quickly. Inhalation causes coughing and discomfort, especially for those with asthma or other breathing problems. Accidental swallowing—maybe a child finding cleaner—causes internal irritation and pain. U.S. Occupational Safety and Health Administration (OSHA) classifies it as hazardous, pushing for gloves and eye protection in workplaces.
In my own experience cleaning offices and coffee machines, a few habits make the difference. I always check labels—if acid sits inside, I grab cheap rubber gloves and safety glasses, especially if I’m pouring powder that can puff out dust. Keeping good ventilation blows away stray particles and helps avoid breathing anything in.
If you’re ever splashed, rinse with water right away—and I mean, just flood it, don’t try to wipe. Skin contact means wash and watch for redness. For the eyes, rinse for several minutes and see a doctor if it stings or your vision blurs. Store this acid above ground, out of reach from curious kids or pets, in containers with tight lids.
Some companies teach safe chemical use with basics like wearing gloves and goggles and never mixing unknown products. Labels translate hazard codes into plain advice. For home users, the main issue happens due to lack of awareness. Safety isn’t about wrapping yourself in a hazmat suit; it’s about respect. A minute’s prep saves hours of regret. If someone doesn’t know what’s in that powder or liquid, a quick search or glance at the safety sheet answers most questions.
In the end, amidosulfonic acid doesn't demand fear; it deserves respect and a little common sense. Treated right, it helps keep homes and machines running smooth, with risks kept well under control.
Amidosulfonic acid, also known as sulfamic acid, shows up in many workplaces, from descaling equipment in water treatment plants to dyeing textiles. This white, crystalline powder looks harmless at first glance, but anyone handling it ought to know just how reactive and potentially hazardous it can be if stored incorrectly. Years spent in industrial environments have taught me that a little prevention goes much further than a dozen emergency drills.
This acid reacts quickly with water. Humidity and spills shorten shelflife, but more importantly, moisture can trigger dangerous fumes. I've seen containers stored close to sinks, windows, or even in bathrooms, which turns an otherwise manageable material into a problem. Keeping it bone dry preserves quality and keeps workers safer, so a climate-controlled, low-humidity room serves best. Any old plastic bag or bent-lidded drum exposes the product to leaks or air, which can lead to unexpected reactions down the line. Sealed, corrosion-resistant drums with gasketed lids create a strong barrier against moisture and outside contaminants. Stainless steel or certain heavy-gauge plastics hold up better than mild steel or cardboard, especially for longer-term storage.
A stuffy, cramped closet may seem out of the way, but poor airflow turns a minor leak into a major hazard. Fumes from amidosulfonic acid feel irritating to breathe, especially in areas where people spend long periods. A well-ventilated storage room means if something goes wrong, the vapors don’t build up. Wherever possible, exhaust fans and vents should be directed outdoors rather than recirculating air inside the facility. My time working alongside facilities managers taught me that regular inspection and maintenance of these systems prevent surprises and keep both regulators and team members more at ease.
One lesson that sticks with me: never store acids near bases, oxidizers, or strong chemicals unless a thick wall or separate room stands between them. Mixing with hypochlorites or strong alkalis can cause violent reactions. Some chemical storage rooms use divider aisles or even dedicated caged shelving to maintain distance. Labels and safety sheets clearly state which substances play well together, but it's practical experience and repeated safety audits that catch near-misses before they escalate.
Label confusion leads to mishaps. Clear, weatherproof labels listing both the chemical name and its hazards ensure new staff and old hands alike can spot issues. I remember a time when a faded label led to an employee trying to clear a spill with a mop, which released noxious fumes and triggered an evacuation. Good labeling practices and restricted access—locked cabinets or badge entry—keep untrained hands away. Only trained staff should access storage areas, as those folks know how to respond if a spill or accident happens.
This acid does not burn, but it releases toxic gases if heated strongly or mixed with the wrong chemicals. That’s why storage well away from heat sources, sparks, or flammable materials makes sense. Spill control kits, including neutralizers, gloves, goggles, and respirators, should stay close by for any unexpected accident. Regular inventory checks and spill drills keep teams prepared for anything.
In my experience, accidents usually come from taking shortcuts. A watchful eye, proper containers, low-moisture rooms, and a culture of clear labeling go further than the latest technology. These habits don’t just protect the chemical’s quality—they protect the folks working with it, their families, and the environment around them. Decades later, stories about careful storage and routine attention still stand out as the best solutions out there.
Amidosulfonic acid shows up in a bunch of cleaning and descaling products, and folks who’ve spent time in labs or on industrial sites probably know its sharp, sour bite. A lot of people don’t realize there’s more at stake with this stuff than just getting it out of the building. This acid can punch holes in metal pipes, chew through clothing, and give skin some serious burns. Tossing it carelessly doesn’t just put the next person at risk. Improper disposal sends harsh chemicals into drains, triggering corrosion in water systems and harming whatever’s living downstream. Even at low levels, this chemical threatens water creatures and damages soil if it makes it to open ground.
Many have the impulse to wash small quantities of chemicals away with water. This shortcut has real-world consequences. Small-scale dumping adds up, as every drain leads somewhere. Water treatment plants aren’t magic: strong acids disrupt biological processes at these plants and corrode infrastructure. Sewer lines aren’t built to handle heaps of acidic material, so persistent dumping may cause leaks, breaks, or even release of hazardous fumes.
Getting rid of amidosulfonic acid safely always means neutralizing it first. Add the acid slowly, bit by bit, to a large volume of cold water. Never the other way around. If things get heated, gases can shoot out and cause splashes or burns. Slowly introduce a mild, basic solution such as sodium bicarbonate (baking soda), keeping an eye on bubbling and fizzing. This reaction is natural as acid meets base. Stop adding base once bubbling dies down and the mixture approaches a neutral pH, around 7. A simple pH test strip or meter provides the surest check. Gloves, safety glasses, and plenty of ventilation offer protection during this whole process.
People working with larger amounts—more than can safely fizz away in the sink—should know this turns into hazardous waste and can’t go with regular trash or down the drain. Every city has different rules, but most local hazardous waste facilities will accept this neutralized solution if you call ahead and explain what's in the container. Labeling containers before drop-off keeps the staff and environment safe. Some companies that generate large amounts set up contracts with professional waste services that handle chemicals from start to finish, making sure nothing harmful escapes.
It’s tempting to consider shortcuts for single-use lab experiments or after a tough cleaning job. That mistake lingers in pipes, affects water treatment workers, and echoes in local waterways. Streams matter to every living thing nearby, from humans down to bugs most folks never see. Smart disposal protects all these connections, and fewer emergencies mean less work for firefighters and environmental crews who already have a full plate.
Clearing out chemicals at home or work often feels like a chore. Calling local hazardous waste programs seems extra, but it saves a neighbor or worker down the line from trouble. Ask the supervisor at work about set disposal plans and where to find safety equipment—not everyone has these answers handy, but it's worth tracking down. Teaching others through example, especially newer lab workers or cleaning staff, spreads safer habits and makes each operation smoother.
Amidosulfonic acid, known by many as sulfamic acid, turns up in everyday cleaning products and some industrial processes. Its formula is H3NSO3, or more commonly, NH2SO3H. This white, crystalline solid doesn’t carry the immediate fame of sulfuric acid or the punch of hydrochloric acid, but its role deserves a closer look.
At the core, amidosulfonic acid brings together an amino group (NH2) with a sulfonic acid group (SO3H). The nitrogen from the amino side links directly with the sulfur atom of the sulfonic portion. A single hydrogen attaches to the amino group, and three oxygens surround the sulfur—one sticks to a hydrogen, making it acidic and reactive, the other two form double bonds with the sulfur atom.
This structure looks much like:
NH2–S(=O)2–OH
I remember my college days pouring tiny crystals into water and watching them dissolve almost right away. Handling it feels a lot less aggressive than sulfuric acid, but you notice the acid bite. Its shelf life stretches far, and it avoids breaking down in air, which gives it an advantage over some of the fuming or unstable acids chemists avoid for routine use.
The arrangement of atoms in amidosulfonic acid changes how it reacts. That direct bond between nitrogen and sulfur makes the molecule solid at room temperature, stable in storage, and easy to handle for cleaning surfaces, descaling pipes, or adjusting pH in pools. The acidic hydrogen on the –SO3H lets it release protons, so it hits stains and limescale with real chemical force.
It doesn’t produce hazardous fumes in the open. It reacts with sodium hypochlorite to neutralize chlorine, which matters in swimming pools or water treatment. For metalworkers and cleaners, this acid sits in a sweet spot. It’s not as nasty as hydrochloric acid, but it still unclogs, descales, and disinfects.
Workers and home users bump into problems with any acid. Amidosulfonic acid can irritate skin, eyes, and lungs. Swallowing it is a mistake, and mixing with strong bases or oxidizers could create heat or toxic gases. Reliable gloves, goggles, and good ventilation cut that risk back to reasonable levels.
Manufacturers have tried to pack it into tablets or granules, and many pool owners prefer it for ease of use. With lower corrosion compared to other strong acids, pipe systems handle it better over time. Still, spills or improper disposal cause trouble for water systems, so collection and proper neutralization matter. Environmental safety grows as a concern, but thanks to its solid, non-volatile form, amidosulfonic acid offers more control in transport and storage.
Amidosulfonic acid works quietly in dozens of industries. You’ll find it in descaling formulas, in some dye and pigment synthesis, and even in laboratories testing for nitrate or nitrite in food and water. Every time it steps in, its chemical structure—one nitrogen, one sulfur, three oxygens, and three hydrogens—keeps it efficient, shelf-stable, and reliable.

| Names | |
| Preferred IUPAC name | Sulfamic acid |
| Other names |
Sulfamic acid Sulphamic acid Aminosulfonic acid Amidosulfonic acid Amidosulfuric acid Aminosulfonsäure |
| Pronunciation | /ˌæm.ɪ.doʊ.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 5329-14-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Amidosulfonic Acid**: ``` NH2S(O)2OH ``` |
| Beilstein Reference | 1713880 |
| ChEBI | CHEBI:29951 |
| ChEMBL | CHEMBL1233241 |
| ChemSpider | 5465 |
| DrugBank | DB03793 |
| ECHA InfoCard | 100.028.769 |
| EC Number | 226-218-8 |
| Gmelin Reference | 63553 |
| KEGG | C01373 |
| MeSH | D001156 |
| PubChem CID | 238720 |
| RTECS number | WO7175000 |
| UNII | JQ27J7B8GK |
| UN number | 2967 |
| CompTox Dashboard (EPA) | DTXSID2022862 |
| Properties | |
| Chemical formula | H3NSO3 |
| Molar mass | 97.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.1 g/cm³ |
| Solubility in water | 213 g/100 mL (20 °C) |
| log P | -2.26 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 0.98 |
| Basicity (pKb) | pKb = 0.28 |
| Magnetic susceptibility (χ) | -53.0e-6 cm³/mol |
| Refractive index (nD) | 1.512 |
| Viscosity | 6 mPa·s (20 °C) |
| Dipole moment | 3.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 99.7 J K⁻¹ mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -534 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -564 kJ/mol |
| Pharmacology | |
| ATC code | V03AB18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye damage, causes skin irritation. |
| GHS labelling | **"GHS05, GHS07, Danger, H315, H319, H335"** |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-0-1-W |
| Autoignition temperature | Autoignition temperature: 530°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 3160 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3160 mg/kg (oral, rat) |
| NIOSH | WX1100000 |
| PEL (Permissible) | PEL: 15 mg/m³ |
| REL (Recommended) | ≥99.5% |
| IDLH (Immediate danger) | 300 mg/m3 |
| Related compounds | |
| Related compounds |
Sulfuric acid Sulfonic acid Sulfanilamide Ammonium sulfate |