Chemists first explored aromatic sulfonic acids back in the 19th century, driven by the dye industry’s hunger for vibrant and colorfast pigments. Around the early 1900s, research into substituted toluene derivatives led to the emergence of 5-Amino-2-Chlorotoluene-4-Sulphonic Acid. The unique structure, featuring a sulfonic acid group and an amino group offset by a chlorine atom on the toluene ring, opened up new routes for the synthesis of azo dyes and pharmaceutical intermediates. Over decades, advances in purification and process optimization have made industrial-scale production a routine affair, helping the intermediates sector meet demand without compromising on safety or environmental considerations.
5-Amino-2-Chlorotoluene-4-Sulphonic Acid shows up as an off-white to light brown crystalline powder. The substance offers high solubility in water owing to the sulfonic acid group, a feature that dye manufacturers find indispensable. Chemists value this compound for its “activated” positions on the aromatic ring, which serves as a canvas for further chemical transformation. It often enters the supply chain bottled tightly to shield it from moisture and contamination due to its hydrophilic nature.
5-Amino-2-Chlorotoluene-4-Sulphonic Acid lays claim to a molecular weight hovering around 223 grams per mole and a melting point situated in the lower 200s Celsius. Its chemical structure features an amino group—ready for diazotization or coupling—accompanied by a chlorine atom that nudges the reactivity of nearby positions on the ring. Acidic and basic functionalities along with the chlorinated aromatic core ensure that this molecule possesses sufficient chemical handle, offering both water-soluble and organic reaction potential.
Industry players ship this substance in leak-proof drums or thick polyethylene-lined bags, complying with the strict packaging guidelines. Typical purity runs upwards of 95 percent as verified by HPLC, with acid-insoluble matter and heavy metal content measured in the single-digit ppm range. Each batch gets a certificate of analysis with a unique batch number, production date, expiry, and hazard communication under globally harmonized standards. Labels warn of potential irritancy and provide UN numbers for regulatory purposes.
The journey of synthesis begins with nitration of 2-chlorotoluene using a nitrating mixture of concentrated nitric and sulfuric acids to generate the relevant nitrotoluene intermediate. Chemists then reduce the nitro group to an amino group, employing iron filings and hydrochloric acid, generating the amino derivative. This is then subjected to sulfonation through treatment with oleum or concentrated sulfuric acid at elevated temperatures, targeting the position para to the methyl group. Extensive filtration, neutralization, and purification follow, ridding the final product of inorganic salts and excess acids.
The reactive sites on 5-Amino-2-Chlorotoluene-4-Sulphonic Acid offer up a world of chemistry. The amino group steps in for diazotization, which leads into a cascade of azo coupling reactions central to dye lab work. The sulfonic acid group keeps the molecule water-friendly, maintaining accessibility for further chemical tweaking. Substitution on the ring, especially adjacent to the chlorine atom, lets chemists introduce other functionalities, customizing the molecule for specialty pigments or pharmaceutical lead molecules. This compound’s versatility underpins its enduring spot in chemical synthesis labs across the globe.
5-Amino-2-Chlorotoluene-4-Sulphonic Acid travels through the industrial marketplace under various monikers. Common synonyms include 2-Chloro-5-Amino-p-Toluenesulphonic Acid and Meta Toluidine Sulphonic Acid. In the dye business, it often turns up as a core ingredient in “FAST” dye intermediates or is tucked away under proprietary codes from local suppliers. These aliases simplify sourcing and cross-referencing, especially across languages and regulatory systems.
Careful handling sits at the center of working with this acid. Safety goggles, gloves, and long sleeves keep skin and eyes safe from its sometimes-irritating dust. Just because a compound looks like harmless white powder does not mean it can be scooped up with bare hands. Occupational exposure limits tend to run tight, and lab ventilation must remain robust. Facilities emphasize closed-system transfers and regular workplace air monitoring to stem the risk of accidental inhalation or chronic exposure. For transport and storage, keeping the compound dry and isolated from strong oxidizers or bases prevents degradation and hazardous reactions.
Synthetic dyes make up the single biggest application space. Here, 5-Amino-2-Chlorotoluene-4-Sulphonic Acid acts as a foundation block for azo dye synthesis, producing vivid reds, oranges, and yellows used to color textiles, plastics, and inks. The water solubility delivered by that sulfonic acid group turns into a big asset for fabric dyeing. Beyond that, some pharmaceutical firms use the acid as an intermediate to build complex molecules for disease treatment, especially where the aromatic amine or sulfonic acid moiety plays a biological role. In the agricultural world, it pops up in the synthesis of certain herbicides and active additives.
In labs around the world, researchers keep poking into new ways to boost the yield and purity of this acid while cutting back on environmental emissions. Greener sulfonation techniques using solid acid catalysts or recyclable solvents show up in the literature. Some teams explore churning out this acid on continuous-flow setups to minimize waste and improve reproducibility. Analytical chemists chase easier ways to detect trace contaminants and polymorphs, smoothing out batch-to-batch consistency. On the frontiers, scientists toy with custom derivatives that might unlock better performance in medical or sensor applications.
Much as the molecule opens doors in manufacturing, it also raises real questions about worker safety and environmental health. Toxicity studies, often conducted on rodents, flag irritation at the eyes and skin along with dose-dependent effects on the liver after repeated ingestion. Standard aquatic toxicity testing points to low persistence and rapid dilution in water, but disposal rules push for neutralization and pre-treatment. The amine group can pose carcinogenic risk if converted to certain aromatic amines, so regulators restrict exposure tightly. Keeping up with this research matters, as regulations and safety protocols keep shifting based on new toxicological profiles.
Shifts in global manufacturing move toward sustainability, driving efforts to trim waste and boost process efficiency across the value chain. For 5-Amino-2-Chlorotoluene-4-Sulphonic Acid, that may signal a move to cleaner synthesis, tighter impurity controls, and better lifecycle management. Demand from the dye industry predicts stability, but researchers keep watching for medical and high-value specialty chemical applications. Growing focus on green chemistry could push new preparation protocols onto the factory floor, while advanced analytics promise finer quality control. Keeping people safe and protecting the planet looms large, but so does the need to keep those bold colors on the world’s fabrics and the innovation pipeline alive in specialty chemicals.
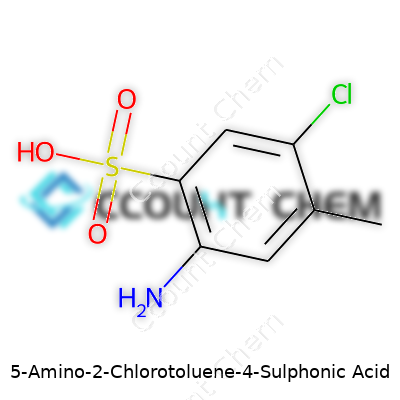
Chemistry sometimes feels like solving a riddle, except the clues follow strict rules. Take 5-Amino-2-Chlorotoluene-4-Sulphonic Acid. The name alone gives hints about what's attached where. Here’s how the structure breaks down: the toluene core, a methyl group stapled to a benzene ring, holds other groups at precise spots—an amino group on carbon number five, a chlorine on carbon number two, and a sulfonic acid group on carbon number four.
Drawing on what I've seen in labs and research papers, this isn’t just textbook stuff. Each group added to that core changes how the molecule behaves. The methyl group boosts hydrophobic character, the amino brings in basicity, the chloro acts as an electron sucker, and the sulfonic acid ramps up solubility in water. These ingredients form the backbone for many dyes and specialty chemicals.
Now for the formula. Take that toluene’s backbone: seven carbons and a methyl group (so that’s C7H8). Add a chlorine (Cl) where the second carbon sits, tack an amino group (NH2) onto the fifth, and bolt a sulfonic acid group (SO3H) on the fourth. The molecular weights and connections line up, giving:
C7H8ClNO3S
Folks working with dyes, pharmaceuticals, or chemical manufacturing want to be certain what's in the bottle, down to each atom. The formula doesn’t only appear as a label. Knowing it lets companies double-check before tossing a new compound into a big batch, hoping for the right shade or effect. Mistakes at this level mean wasted time, wasted cash, and can even risk safety.
Remember my days working with process engineers aiming to reduce costs? One misstep mixing the wrong chlorinated or sulfonated aromatic resulted in gallons of off-color, unusable product. Catching that error early by confirming the formula sidestepped hours of troubleshooting. Beyond big industry, clear molecular details keep consumer goods—from synthetic textile dyes to cleaning agents—safe and effective.
It’s easy to overlook, yet the chemical formula shapes everything from hazard assessments to regulatory paperwork. Under modern rules through the EPA or REACH, companies prove safety and track every component listed by its formula. The right numbers mean avoiding fines, keeping shipments clear at customs, and maintaining consumer trust.
Plenty of folks focus on applications like watercolor brilliance or how tough a molecule can get under high heat. None of that works out if the composition goes unchecked. Whether in university research or on-the-ground formulation, accuracy in composition directs every step.
A formula like C7H8ClNO3S does more than fill a textbook page. It signals scientists’ collective effort to keep pushing boundaries—toward greener production or safer material use. Building on reliable, detailed molecular data, researchers and manufacturers keep moving chemistry from the lab to the everyday world with fewer surprises and smarter solutions.
Step into any textile factory, and chances are you’ll find 5-Amino-2-Chlorotoluene-4-Sulphonic Acid somewhere in the workflow. This compound shows up as an intermediate in the production of azo dyes, which you might recognize from the colors in clothing, upholstery, and even stationery. The textile world relies on such intermediates for stable color, good resistance to washing, and consistency across large batches. Without reliable intermediates, dye-makers would face huge setbacks matching colors or keeping fabric shades bright over time.
The appeal of this amino sulfonic acid doesn’t stop at basic color. Its structure allows chemists to build more complex dye molecules with better performance. The amino and sulfonic groups play specific roles, letting dye chemists target fibers like cotton or wool with greater selectivity. Textile mills benefit because their products stand up to sunlight and repeated cleaning. That matters to people like me who’ve worn faded shirts and wondered why some colors last while others look washed-out after a few spins.
The story of this chemical extends past the textile factory. In the pigment and ink industry, manufacturers use derivatives from the same family of compounds to make printing inks and pigments for plastics. Designers in packaging or advertising use these colors to catch the eye or deliver a brand message that’s easy to spot on store shelves. Because of its performance in light and heat, scientists keep picking these intermediates as a base when formulating new pigments for tougher jobs, such as outdoor signage and automotive coatings.
Widespread industrial use brings questions of safety and environmental impact. Factories that handle 5-Amino-2-Chlorotoluene-4-Sulphonic Acid must train their workers, watch for spills, and comply with rules for effluent treatment. Nobody wants dye pollution running into streams, staining rivers and harming ecosystems. In places where local governments keep a close watch, strict wastewater treatment keeps this risk in check. Global companies look for safer alternatives or invest in closed-loop recycling to recover unused chemicals and reduce waste.
Dye production still depends on a handful of intermediates made from petrochemical sources. Sustainable chemistry is changing the picture, slowly. I’ve spoken with researchers and plant managers who say that investment in greener alternatives depends on market demand and regulations. When big buyers push for safer, eco-friendly dyes, chemical firms have a reason to explore plant-based sources or cleaner processes. Pilot projects have started showing that plant-derived aromatics can partly replace synthetic routes, though challenges around cost and quality remain.
For those working in textiles, printing, or chemicals, transparency about raw material sourcing and waste management keeps everyone accountable. Industry groups have begun sharing best practices and working with environmental agencies to improve standards. Giving workers proper training and upgrading waste processing plants pays off, not just for water quality, but for a brand’s reputation in today’s market. Strong partnerships between manufacturers, regulators, and researchers make it possible to balance production needs with environmental responsibility.
Experience with chemicals over the years has shown that certain compounds look harmless at first glance, but ignore their risks and trouble waits. This rings true for 5-Amino-2-Chlorotoluene-4-Sulphonic Acid. It’s a powder, sure, but even a dust can get into the air or find its way onto unprotected skin, causing a burning sensation or an irritating rash. Breathing those fine particles can lead to coughs and sneezing, sometimes even worse respiratory effects. Long-term exposure in some factory settings has led to chronic skin issues, and in very rare cases, more severe toxicity.
Gloves aren’t all the same. Nitrile or butyl rubber gloves usually do the job here, since this acid can bite through thinner materials over a long shift. A good pair of goggles brings peace of mind because stray dusts and vapors have no respect for eyes. Closed shoes and long sleeves are standard. Lab coats soaked with chemical dust will only rub contaminants into skin, so aprons help, especially for jobs that last more than a couple of hours.
Old labs or plant rooms often lack the air movement newer sites enjoy. It’s easy to forget the difference a working exhaust fan can make until the air inside becomes hard to breathe. Fume hoods pull vapors and dust out before lungs can grab them. Even a portable filter or simple open window reduces risk, especially in smaller spaces. Dust masks filter out most inhalable bits — a simple change that saves a lot of sneezes and inflammation down the line.
Spills happen, fatigue sets in, and lids sometimes get left ajar. Acid left exposed soaks up room moisture and clumps, making cleanup harder each passing hour. I keep everything in tightly closed containers, preferably glass or high-density plastic, stored cool and dry, with clear labels that make sense to the next shift as much as to myself. One forgotten jar near a heat vent can turn into a headache, or worse, a chemical release into the room.
Plenty of people think a splash of water cleans everything up, but with an acid like this, a quick rinse only spreads the contamination unless gloves are on and waste gets contained. Neutralizing spills with sodium bicarbonate helps. Shovels, not bare hands, scrape up any piles. Soiled clothing comes straight off after a spill, bagged and laundered separately. Emergency eyewash stations and showers should be within a short stride from workbenches. Training new staff on emergency steps isn’t just a box to tick — practice builds the reflexes people actually lean on when things go wrong.
Records from occupational safety groups show that chemical contact injuries drop by over fifty percent in labs that stick with personal protection, sealed containers, and good air flow. Putting real money into proper training and labeling has saved both health and budget for countless organizations. Safety folk used to say, “One mistake makes a memory, and not a good one.” With chemicals like this, memories ought to come from a job done well, without a hospital visit or long-term scars.
5-Amino-2-chlorotoluene-4-sulphonic acid isn’t a name you run into at the supermarket. This compound, rooted in aromatic chemistry, often pops up in dye manufacturing, specialty chemicals, and research. In my lab experience, the first time I reached for this crystallized powder, the goal was straightforward: see how easily it would dissolve—because nothing clogs a process faster than a stubborn precipitate.
Drop this acid into a beaker of cold water and patience gets tested. It doesn’t dissolve well at room temperature—users report only moderate solubility, and even that demands some stirring and, occasionally, warmth. The benzene ring and chlorine atom don’t cooperate with water’s desire for hydrogen bonding. I found using hot water or even mild heating helps break it up, but residue can remain if concentrations climb. For chemistry nerds, its solubility hovers at just about 10-20 grams per liter, which puts a squeeze on large-batch processes.
Turn to polar organic solvents, and results vary. Ethanol offers little joy; the acid’s sulfonic group doesn't want to leave its ionic comfort zone in water for the less polar embrace of alcohol. Acetone and ether barely move it. DMSO and DMF can dissolve it far better, thanks to their polar aprotic character and ability to disrupt intramolecular bonding. I recall switching to DMSO on a stubborn synthesis round, and suddenly a cloudy suspension turned clear.
Solubility doesn’t feel glamorous, yet it affects every step in preparing dyes, reagents, and pharmaceutical intermediates. If you can’t get an even solution, reactions limp along or fail, material gets wasted, filters clog, and costly downtime piles up. Poor solubility also means more energy spent—heating and mechanical stirring—which raises costs and environmental footprint. I’ve seen small dye setups grind to a halt and lose product simply because a water-based dissolution step got misjudged.
Textbooks back up the bench experience: sulfonic acids often find water a grudging host, especially with added hydrophobic rings and halogens. Industry safety sheets peg solubility as low at ambient temperature, echoing the lab struggle. For process engineers, detailed studies haven’t always surfaced—a gap that can surprise newcomers who expect "sulfonic acid" to be shorthand for "very water-soluble." That’s not always true.
If your setup depends on using this acid, maximizing surface area and moderate heating gives the best results in water. Ultrasonic agitation has sped up my dissolutions in a pinch, breaking up stubborn clumps. Picking the right solvent sometimes means shifting to DMF or DMSO for complete solubilization, though their toxicity and disposal costs can’t be ignored. Teams in industry might benefit from investing in small-scale solubility testing before scaling up, instead of relying on scant literature data or assumptions.
You can’t overlook substance solubility. It’s easy to miss on a data sheet, but in practice, it shapes your choice of chemicals, energy costs, and long-term sustainability in manufacturing and labs. Next time a project log jams at the dissolution stage, a closer look at solvent compatibility, temperature, and mixing might turn frustration into progress.
Years in the lab have taught me that the right storage for specialty chemicals isn’t just a detail—it’s essential for research integrity and workplace safety. When bottles arrive with complex labels like 5-Amino-2-Chlorotoluene-4-Sulphonic Acid, I always pause to review the storage requirements. Science isn’t forgiving if a material degrades or reacts because someone tucked it onto a sunny shelf or next to incompatible compounds.
5-Amino-2-Chlorotoluene-4-Sulphonic Acid, used in making dyes and colorants, doesn’t announce when it’s about to lose quality. Moisture or heat creep in, and suddenly the chemical changes—sometimes the color shifts, other times the compound clumps or gets sticky. Degraded material wastes time and money, and I’ve seen more than one experiment fizzle because someone grabbed an old, improperly stored batch. Even health can be at risk, since decomposition might produce dust or vapors nobody in the lab wants to inhale.
Based on chemical safety data and my own experience, keeping 5-Amino-2-Chlorotoluene-4-Sulphonic Acid in a cool, dry, well-ventilated area pays off. Room temperature may sound fine, but climates differ—so I always recommend a controlled environment below 30°C. Excess moisture is trouble for sulfonic acids. I’ve opened containers that were left out in a humid storeroom, only to find a solid that had turned to paste, with visible caking. Sealed, air-tight containers make a difference. Polyethylene bottles with robust screw-caps or glass containers with chemical-resistant liners work best—simple plastic bags never cut it.
I’ve noticed too many labs save on space by mixing incompatible chemicals on crowded shelves. Reactive contaminants are a hidden hazard, especially near acids and bases. I never shelve aromatic amines near oxidizing materials. Separate cabinets for organics and strong oxidizers avoid surprise reactions and unwanted breakdown. Labels help, but routine training for the team keeps everyone on the same page and turns good storage into a habit, not an afterthought.
Direct sunlight speeds up chemical decay. One summer, we realized part of our storeroom caught the late afternoon sun, and we lost half a batch of sensitive dye intermediates. Opaque bottles plus a shaded, indoor space reduce that risk. I keep chemicals off the floor, away from heating vents and cold drafts, since temperature swings stress packaging and shorten shelf life. Regular inventory checks help catch outdated or compromised containers before mistakes happen in the lab.
Proper storage also means preparing for the end of a chemical’s life. I keep a clear disposal protocol for expired or degraded materials. Having a logbook for chemical receipt dates, batch numbers, and storage conditions gives me peace of mind. By tracking every movement, I spot problems early—before they affect the next experiment or compromise anyone’s safety.
Consistently safe and effective research comes from small choices, like careful storage of sensitive substances. In my experience, well-maintained stocks support reliable results and create a safer workplace for everyone involved. Anyone who works with 5-Amino-2-Chlorotoluene-4-Sulphonic Acid knows the effort pays off—not only for quality outcomes but for credibility in every report, batch, and discovery.
| Names | |
| Preferred IUPAC name | 4-Amino-5-chloro-2-methylbenzenesulfonic acid |
| Other names |
2-Chloro-5-amino-4-methylbenzenesulfonic acid 4-Methyl-2-chloro-5-aminobenzenesulfonic acid 2-Chloro-5-amino-p-toluenesulfonic acid 5-Amino-2-chloro-4-methylbenzenesulfonic acid |
| Pronunciation | /faɪ-əˈmiːnoʊ-tuː-kloʊˈriːn-tɒlˈjuːiːn-fɔːr-sʌlˈfɒnɪk-ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-44-8 |
| 3D model (JSmol) | `3D model (JSmol)` string for **5-Amino-2-Chlorotoluene-4-Sulphonic Acid**: ``` CC1=C(C=C(C(=C1N)S(=O)(=O)O)Cl) ``` |
| Beilstein Reference | 2856806 |
| ChEBI | CHEBI:28448 |
| ChEMBL | CHEMBL1876691 |
| ChemSpider | 160028 |
| DrugBank | DB14184 |
| ECHA InfoCard | 17-211-120-0 |
| Gmelin Reference | 90398 |
| KEGG | C14322 |
| MeSH | C09-4632 |
| PubChem CID | 156168 |
| RTECS number | GV8750000 |
| UNII | 4XV6A2VE1L |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID2039246 |
| Properties | |
| Chemical formula | C7H8ClNO3S |
| Molar mass | 207.67 g/mol |
| Appearance | Light yellow powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -0.13 |
| Vapor pressure | 0.0 mmHg at 25°C |
| Acidity (pKa) | pKa ~ -2 (sulfonic acid group) |
| Basicity (pKb) | 5.18 |
| Magnetic susceptibility (χ) | -6.1 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Dipole moment | 3.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.96 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -458.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1297.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | 'H302, H315, H319, H335' |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| Flash point | Flash point > 100 °C |
| LD50 (median dose) | LD50 (oral, rat): 5000 mg/kg |
| NIOSH | RN1481 |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
5-Amino-2-chlorotoluene 2-Chloro-5-nitrotoluene Toluene-4-sulfonic acid 2-Chlorotoluene-4-sulfonic acid 4-Amino-2-chlorotoluene |