Chemicals with a legacy often reveal the progress of both science and society. 4-Toluenesulfonic acid, also known as TsOH, has roots dating back to the turn of the 20th century. Early researchers discovered its power as an acid catalyst during a period when chemists struggled with balancing reactivity and selectivity in organic reactions. In the decades that followed, the compound’s monohydrate form became a laboratory staple, valued because it delivered consistent reactivity and easier handling compared to volatile mineral acids. By the 1950s, TsOH monohydrate found its place in textbooks and chemical catalogues, helping generations of students and industrial technicians understand acid-catalyzed transformations in synthetic chemistry. Its history ties directly to the growing adoption of organic synthesis in pharmaceuticals, plastics, and specialty materials. The steady rise in demand for reliable, manageable acids cemented its role as a workhorse in both research institutions and factories.
This compound, usually provided as a white, crystalline solid, bridges the needs of fine chemical synthesis and largescale manufacture. Chemists turn to 4-Toluenesulfonic acid monohydrate because the dry form provides straightforward weighing, storage, and transport. It’s easy to spot by its faint odor and deliquescent quality – the ability to attract moisture from the air is one of its defining quirks. Many labs keep it close at hand for dehydrations, acylations, or even polymerizations, since it rarely surprises with side reactions or tricky byproducts. Thanks to its non-volatile nature, companies avoid some of the challenges that come with more hazardous or fume-producing acids, streamlining operations for both routine and advanced projects.
4-Toluenesulfonic acid monohydrate has a chemical formula of C7H8O3S·H2O. It melts at around 103-104°C, transforming from solid to a syrupy liquid before decomposing at higher temperatures. The density sits near 1.24 g/cm³, which makes it pack solidly but not overly dense for handling or mixing. Its solubility in both water and polar organic solvents provides immense flexibility, supporting a wide range of synthetic conditions. The crystal structure helps suppress dusting, lowering the risk of inhalation. Acidity stands out, with a pKa near -2.8—much like sulfuric acid—though the lower volatility adds extra reassurance. Its sulfonic acid group is the powerhouse behind deprotonation, catalysis, and nucleophile activation, driving countless reactions in organic chemistry.
Manufacturers deliver TsOH monohydrate in a spectrum of purities. Reagent or ACS-grade material meets tight limits on heavy metals, halides, and moisture. Typical labeling covers CAS number 6192-52-5, along with standardized warnings about skin, eye, and respiratory contact. Most suppliers use humidity-resistant packaging, since prolonged exposure to air causes caking and degradation. Labels highlight the need for gloves, goggles, and dust control equipment on the shop floor or in teaching labs. Certificates of analysis accompany every batch, outlining titratable acid strength and confirming the absence of troublesome impurities. This transparency, dictated by both national and international standards, helps organizations remain in step with increasingly strict environmental and worker safety rules.
Synthetic chemists make this compound by reacting toluene with sulfuric acid, typically through sulfonation. The product, toluenesulfonic acid, is then carefully crystallized from aqueous solution, often followed by drying under controlled humidity. A final hydration step ensures the monohydrate form, capturing precisely one water molecule per TsOH unit. This water of crystallization keeps the crystals stable and free-flowing—a useful feature for everything from chemical scale-up to educational demonstrations. Factories streamline energy use and waste handling by recovering much of the spent sulfuric acid, reducing both cost and environmental burden. This process, refined through years of manufacturing experience, demonstrates the mix of precision and pragmatism that defines industrial chemistry today.
In the lab, TsOH monohydrate consistently delivers as both a strong acid and a handy catalyst. Synthesis of esters, ethers, and other building blocks benefits from its sharp acidity and clean reactions. TsOH’s non-oxidizing character means it rarely introduces unwanted side chemistry—an advantage in delicate organic transformations. In peptide synthesis, TsOH reliably removes protecting groups with almost no risk of racemization. Beyond that, its sulfonate group acts as a leaving group in nucleophilic substitution reactions, supporting both simple conversions and complex, multi-step syntheses. Modifications to the core TsOH structure, such as converting to its sodium salt (p-toluenesulfonate), help expand its use into detergents, dyes, and electroplating. Its broad compatibility makes it a favorite for researchers testing new reaction pathways or process engineers troubleshooting large-scale production.
Over the years, chemists have used many names for this staple. p-Toluenesulfonic acid monohydrate sits alongside synonyms like para-toluenesulfonic acid monohydrate, p-TsOH·H2O, and 4-methylbenzenesulfonic acid monohydrate. In some contexts, the name “TsOH monohydrate” appears on technical literature, safety data sheets, or bottle labels. Some vendors reference Brand-Specific designations, but the chemical identity stays clear due to meticulous labeling and harmonized product codes. This roster of names occasionally confuses newcomers but rarely stumps experienced personnel or educators who follow the history and evolution of chemical nomenclature.
Handling any strong acid brings inherent risks, and workers learn to respect TsOH monohydrate for its corrosive bite. Direct contact causes burns or irritation, mandating protection for eyes, skin, and airways. Facilities adopt rigorous protocols: chemical-resistant gloves, face shields, and ventilation are just the start. Automated systems now measure and transfer more hazardous reagents, shielding people from spills or accidental exposure. Emergency measures, including eye wash stations and clear first aid signage, reduce the seriousness of any mishap. Companies looking after their people train new hires repeatedly in chemical hygiene and maintain tightly controlled access to reagent stores. As regulatory agencies tighten oversight, the habits and equipment evolved to manage TsOH monohydrate serve as templates for other potentially dangerous compounds. Continuous education on Material Safety Data Sheets and best practices helps cut down both accidents and chronic health concerns.
This acid crosses boundaries between academic research and heavy industry. In pharmaceuticals, TsOH monohydrate supports active ingredient synthesis, especially for complex molecules where water-sensitive catalysis matters. Manufacturers rely on it for making specialty plastics, resins, and coatings with precise molecular weights and tailored performance. Lab instructors teach budding chemists about catalysis, esterification, and elimination reactions using TsOH as an example of a strong, reliable acid—easy to handle but powerful in action. Art conservation uses its cleaning power, removing stubborn deposits without damaging artwork. Water treatment leverages its sulfonate group to modify ion exchange resins, improving selectivity and durability for municipal supplies. Fine chemical and flavor industries turn to TsOH for efficient transformations, keeping batch costs low and minimizing side products. I’ve seen its value firsthand, both in undergraduate labs and high-throughput industry settings, where productivity hinges on trustworthy, straightforward reagents.
Every decade sees a new wave of applications and improvements. Recent R&D pushes focus on greener, more sustainable synthesis routes, limiting high-energy or high-waste aspects of production. Chemists engineer selective catalysts based on TsOH to run faster, cleaner reactions using less solvent or milder conditions. New composite materials exploit the acid’s ability to fine-tune conductivity, solubility, or adhesion. Biomedical researchers explore modified sulfonates in targeted drug delivery or diagnostic imaging. Its high willingness to engage in hydrogen bonding spurs interest in supramolecular assemblies and “smart” materials sensitive to environmental changes. Looking ahead, process engineers experiment with combining TsOH-based systems with automation, driving up yields while cutting errors. Research into less irritating derivatives, or immobilized TsOH versions anchored to polymers, aims to deliver power with added safety and even easier cleanup.
Toxicological studies show that p-toluenesulfonic acid, even in its monohydrate form, earns its hazard pictograms. Contact can cause skin or mucous membrane burns, eye damage, and respiratory tract irritation. Animal studies report acute effects mostly related to this acid action, not systemic toxicity, unless exposure is extremely high or persistent. Chronic exposure hasn’t revealed strong carcinogenic or teratogenic tendencies, but sorting risk in industrial settings demands more than just animal data—regular human exposure monitoring and clear hazard communication matter a lot more on factory floors and in busy research environments. Ongoing research focuses on both acute effects and the chronic impact of sub-lethal exposures, especially as manufacturing scales up and as waste management tightens under global environmental agreements. Responsible disposal, using neutralization and professional waste handlers, forms a core plank of any safety regimen.
Chemistry grows by building on reliable tools, and 4-toluenesulfonic acid monohydrate keeps earning its spot thanks to unmatched stability and consistent performance. Green chemistry pushes will ask suppliers for cleaner production and easier recycling, blending classic processes with new digital and sustainable tools. Custom formulations—maybe granules with color-coding for easy tracking, or immobilized blends that cut down airborne dust—could make the compound even friendlier in automated facilities or teaching labs. The pace of change in pharmaceuticals, electronics, and environmental cleanup hints at new uses for powerful, targetable acids like TsOH. Digital monitoring of chemical stocks, tighter tracking of user exposure, and machine-assisted transfer and weighing promise a future where power and safety go hand in hand, giving every lab and plant access to the best of both worlds.
Anyone working with chemicals in a lab has probably run into 4-Toluenesulfonic acid monohydrate (sometimes people just call it pTsOH). I’ve handled it myself—I remember my first year in a synthetic organic chemistry lab, a senior researcher tossed me a container and reminded me to keep it away from water unless I wanted a mess. This compound carries more weight in labs and manufacturing than most realize.
In the world of chemistry, researchers often need acids strong enough for a job, but less fussy than sulfuric acid. 4-Toluenesulfonic acid monohydrate answers that call. Folks running esterification or acetal formation reach for it because it’s strong and easy to measure out. Trying to build new molecules from scratch? Adding pTsOH to the mix gives a controlled acidic environment, shaving off hours, sometimes days, compared to weaker options.
The monohydrate form, which includes a single molecule of water, grabs attention because it’s less likely to clump or cake in storage—lab techs waste less material and see fewer failed syntheses. On a rough day, just this detail meant the difference between hitting a research deadline and starting over.
Outside of labs, the story keeps going. In the pharmaceuticals world, pTsOH pushes forward key steps in making medicines. Without it, some painkillers or antibiotics would cost more, take longer to make, or wouldn’t come out with the same purity. Friends working in mid-sized pharma sweat over reaction yields; pTsOH’s reliability often gets a quiet nod of thanks when a batch meets quality targets.
Dye makers, too, use it for creating brighter, longer-lasting colors. Without the right acids, those reds, blues, and greens on your favorite clothes or home décor would fade faster, and a lot more material would end up wasted in the process. The economics of textile production tighten with every cent saved, so a dependable acid agent keeps jobs and invests directly in local economies.
Few think about what happens if something goes wrong with the chemicals behind the scenes. Inconsistent batches or poorly handled acids can slow an entire pharmaceutical facility or flood local rivers with waste. Ethical labs use pTsOH because it breaks down easily and rarely leaves hard-to-clean residues compared with older alternatives like sulfuric acid or hydrofluoric acid. Less hazardous waste gets produced. This control stretches from environmental impact all the way to worker safety. Trust grows when employees know leadership considers the chemicals on hand, which isn't always true in some industries.
Though pTsOH solves a lot of problems, chemists and policy makers can push things further. Educating lab techs on storage and safe handling still means fewer emergencies. Sharing new findings—like better neutralization methods or using greener solvents—spreads responsible habits fast. Supply chain traceability and responsible disposal policies help keep trust alive between manufacturers, researchers, and the public.
Practical chemistry isn’t just about mixing stuff. The choice of something as humble as 4-Toluenesulfonic acid monohydrate reminds us how each bottle in a cabinet reflects real priorities: safety, quality products, and respect for the environment and people behind every batch.
Anyone handling chemicals learns quickly: storage is as important as the process itself. I remember my early days in the lab. Our mentor would look over her glasses and scold anyone who left a bottle of acid on the wrong shelf. 4-Toluenesulfonic Acid Monohydrate earned particular attention. In a world that relies on clean reactions and safe environments, this white, crystalline substance can quickly cause problems if left in the wrong place.
This compound attracts moisture from the air. Even a small slip—an untightened cap, a damp benchtop—turns a stable reagent into a sticky mess. My own experience taught me how minor neglect ruined a batch of product. Moisture changes the purity, and for those of us pushing for reliable data, every bit of contamination throws off the results. Fact: moisture creates clumps, alters weights, and—left unchecked—can trigger slow decomposition.
Beyond water, 4-Toluenesulfonic Acid Monohydrate reacts strongly with bases and oxidizing agents. I watched a colleague chase a spill that happened near a bottle of sodium hydroxide; the reaction wasn’t explosive, but it smoked and released irritating fumes. It doesn’t take much to set off an unnecessary emergency.
Good storage starts with dryness. Store 4-Toluenesulfonic Acid Monohydrate in a tightly closed container. I’ve seen people use wide-mouth jars with loose-fitting lids—they ended up tossing half their stock each year. Only airtight, chemical-resistant containers stand up to humidity. Polypropylene and glass containers with solid, screw caps work well. I add a packet of desiccant to keep things bone-dry, a trick picked up from a seasoned technician who never lost a single gram to clumping.
Coolness helps. Room temperature storage works best. Shelves pitched too close to heat vents or windows bring trouble: temperature swings pull moisture right out of the air. My own lab moved sensitive materials away from sunny spots; loss rates dropped, and sample quality improved. Never freeze the compound, though. Special chemical refrigerators keep reagents safe, but extreme cold doesn’t suit this acid. Below-average temperatures can trap condensation inside the bottle once it’s opened.
Location requires attention to detail. Never store acids next to bases, oxidizers, or materials that generate heat. Label everything with the exact chemical name and date you opened it. In a crowded chemical cabinet, carton boxes and plastic bins never replace a proper segregated shelf. Flammable storage cabinets are a bad match here; acids need their own real estate. Spacing matters, too—overcrowding means you can’t keep leaks or spills contained.
I’ve seen too many students fumble with poor habits and poor checks. Forgetting to check the condition of the powder before measurements led to failed syntheses more than once. It pays to look—clumpy, off-color, or sticky samples mean trouble. Throw those out.
At the end of the day, proper storage of 4-Toluenesulfonic Acid Monohydrate comes down to being vigilant, organized, and cautious. Avoiding water, keeping it cool and dry, storing it away from reactive chemicals and heat, and labeling it clearly will keep the lab – and anyone working in it – on the safe side. Paying attention to storage saves time, money, and peace of mind.
You probably won’t find a bottle of 4-Toluenesulfonic acid monohydrate sitting next to your baking soda at home, but it shows up a lot behind the scenes in labs and factories. Chemists use it all the time to push along reactions or to clean up old chemical mixtures. Some folks think it’s just another acid on the block, not as scary as the classic strong acids you see melting rubber gloves in cartoons. Truth is, it holds its own kind of risk that’s easy to miss if you don’t look up the safety sheet or chat with someone who works with the stuff.
Most people hear “acid” and think of burns. They’re not wrong—spilling this compound on your skin feels a lot like getting splashed with battery acid. I’ve seen red, irritated arms after careless handling, and nobody enjoys explaining burns to the safety manager. It stings eyes and airways too, especially when someone gets lazy with the mask or fumbles the container and releases dust or mist into the air. After a busy day in a cramped lab, a loose lid can leave you coughing or blinking back tears.
Besides the quick pain, there’s the issue of what happens if you breathe in or swallow it. I wouldn’t wish that stomach pain or throat burn on anyone. Once, a coworker got a lungful during a spill. He felt like he’d run a marathon for days. Chronic exposure has its own game—a slow grind on your health, including breathing trouble that can sneak up after years of sloppy habits.
We can’t ignore what happens to this acid when it leaves the lab. While not topping the charts for environmental nightmares, dumping it down the drain isn't smart. It’s acidic, it eats away at pipes, and it can skew the pH of waterways. Even small changes in water acidity mess with fish and plant life trying to survive downstream. Proper chemical disposal rules exist for a reason—sometimes it’s tempting to take shortcuts, but those actions snowball fast, especially for students and new lab techs just trying to clean up before lunch. Responsible disposal means keeping chemicals like this out of the ecosystem and away from kids or pets.
Staying safe around this acid means nailing down habits, not just memorizing rules. Every experienced lab worker has a story about someone who thought gloves and glasses were optional until reality hit. Good training beats long lists of instructions written in tiny print. A proper fume hood, a face shield when mixing solutions, and gloves you’d trust for hot dish duty all make a real difference.
People sometimes skip steps when they’re in a rush, especially if they think this compound is “less dangerous” than bigger-name acids. I’ve found it helps to talk through the “why” behind every safety step. If you know a chemical burns or corrodes on a molecular level, you’re much more likely to snug those goggles and double-check your tools before getting started.
Manufacturers, supervisors, and teachers play a big role here. A well-stocked supply closet, a clear spill plan on the wall, and encouragement to speak up about near-misses make safer labs and workplaces. Open conversations about chemical safety create trust and catch mistakes before they turn into emergencies.
4-Toluenesulfonic acid monohydrate does a lot of useful things behind the curtain of modern chemistry. Getting careless with it can lead to burns, coughing fits, or bigger health problems over time. Respecting the hazards means building good habits and treating even routine substances with the caution they deserve. Simple changes in attitude and training do more for safety than any stack of warning labels. That’s how you keep tough chemicals from turning everyday work into a hazard.
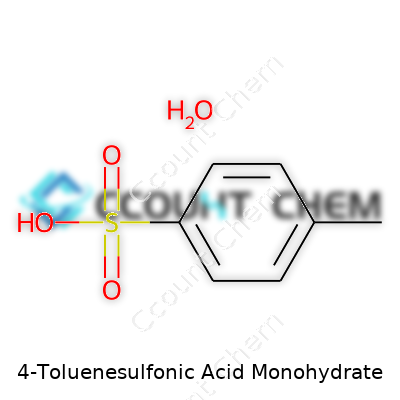
Plenty of folks roll their eyes at the intricacies of chemical formulas. The question of what exactly 4-Toluenesulfonic Acid Monohydrate is might seem like a narrow corner of the chemical universe, but clarity on this topic helps bring trust and understanding in lab settings and manufacturing plants alike. The chemical formula for 4-Toluenesulfonic Acid Monohydrate clocks in as C7H8O3S·H2O. A mouthful, sure, but each part indicates something very real: you’ve got seven carbons, eight hydrogens, three oxygens, a sulfur, plus a water molecule—that “monohydrate”—tucked in the crystal structure.
Plenty of experiments go wrong for want of pure material, and a surprising number of headaches start with basic misunderstandings about chemical composition. A lab-grade sample means you get the stated monohydrate, not a mix or anhydrous form, so the formula does more than impress teachers. It proves to purchasers and researchers that the necessary hydration state is present—something crucial in organic synthesis. In the pharmaceutical world, for instance, even a trace amount of extra water left from the monohydrate could throw off product stability, change how a compound dissolves, or skew yields. From my own grad school work, more than one late night was spent trying to track down why a reaction refused to behave, only to realize the hydrate content had shifted on the shelf.
There’s a reason big names like Sigma-Aldrich or Merck list the monohydrate variant strictly—chemists across the globe expect the water of hydration to be present and accounted for. The sulfonic acid moiety attached to the toluene ring gives the compound a strong acid punch. People rely on its performance as a catalyst in esterification and acetalization, making it a staple in both small-scale research and large-scale production lines. According to peer-reviewed studies, inconsistent product labeling or hydration states can cut yields or impact purity by several percentage points—costly mistakes in tight-margin industries.
Too many non-chemists feel out of their depth looking at these chemical names, but every component tells a story about reactivity, use, and safety. For those creating safety data sheets (SDS), a slip in naming can have real-world consequences if first responders or handlers don’t realize water is present in the structure. Handling protocols, storage conditions, or spill clean-up all rely on accurate formula details. Chemists and supply chain professionals working together, supported by regulatory oversight and certification, keep mishaps to a minimum.
Moving forward, clearer product labeling and traceability bolsters trust. Barcode tracking and blockchain for chemical supply have already begun to roll out in some regions—and for good reason. Automated spectrometry or water-content testing prior to use can spare labs headaches and wasted resources. Laboratories benefit from up-to-date training that doesn’t just skim formulas but digs into how errors ripple through experimental and quality outcomes. Trust and accuracy grow from routine checks and honest documentation, forming the backbone of research and industry.
Knowing the chemical formula for 4-Toluenesulfonic Acid Monohydrate isn’t just trivia. It streamlines research, strengthens safety, and helps everyone—chemist or not—make decisions with confidence. The more time spent getting these details right at each step, the fewer surprises pop up down the line.
There’s a reason safety sheets for 4-Toluenesulfonic Acid Monohydrate tend to look like stern warnings. This compound brings muscle in chemical synthesis, helping folks in labs and factories build all sorts of useful products. It doesn’t mix well with skin, eyes, or lungs. Anyone who’s worked with strong acids knows the sting, the cough, and the general frustration brought by a small mistake.
Lab gloves, protective eyewear, and a real lab coat (not just your old jacket) form the basic defense. I remember an old mentor telling stories of people brushing off goggles, only to spend an afternoon washing chemical splash from their lashes. The burns fade; the lesson usually sticks. Long sleeves and closed shoes help too. Good ventilation tops off that armor—never underestimate the value of a working fume hood.
4-Toluenesulfonic Acid Monohydrate gets along best with dry air, room temperatures, and sealed containers made of glass or high-quality plastic. Moisture in the air wants to pull it out of its container and onto countertops. A tightly closed lid, clear labeling, and a dedicated spot away from bases and oxidizers work wonders. I’ve seen spills caused by mixing incompatible chemicals, sometimes from nothing more than a lazy shelf arrangement.
The urge to grab paper towels and “just wipe it up” causes more harm than good. Spills call for an absorbent material that handles acids (not all chemical spill kits work equally well). Once absorbed, the mix slides straight into a labeled and compatible waste bin. Running to the sink tempts folks who want quick solutions, but water washing acid toward the drain only causes trouble down the line.
Tossing 4-Toluenesulfonic Acid Monohydrate in the regular trash won’t fly, legally or ethically. This goes double if the chemical touched solvents or got mixed in synthesis waste. Proper waste containers, typically plastic lined with acid-proof bags, become essential. Label everything—nobody wants to play guessing games with chemical waste later on. I’ve watched coworkers scramble to identify a mystery container more than once, and the extra paperwork always stings.
Local environmental laws spell out how to handle chemical waste. Registered disposal companies do the heavy lifting, picking up well-sealed drums and logging every detail. Documentation isn’t just a formality. It keeps fines and reputation hits at bay, and it protects the crew who deal with hazardous waste each week.
Training plays a huge role. Every staff member should know where the safety data sheet lives and what to do in case of a spill or accidental contact. Regular drills and refreshers save nerves (and sometimes skin). For anyone who manages chemicals day in and day out, careful habits become second nature. It’s tempting to cut small corners, but the stories of those who didn’t provide daily reminders to show respect for every bottle on the shelf.
Easy-to-read labels, smart storage, real-time safety reminders, and accessible spill kits make all the difference. New staff should shadow experienced workers, picking up tricks and caution from those with years on the job. Reporting every slipup without blame helps the next group learn, and it builds trust in the lab or plant. Protective gear, regular training, and knowing the local rules keep both workers and the environment safer every day.
| Names | |
| Preferred IUPAC name | 4-methylbenzenesulfonic acid monohydrate |
| Other names |
p-Toluenesulfonic acid monohydrate p-TsOH monohydrate Para-toluenesulfonic acid monohydrate 4-Methylbenzenesulfonic acid monohydrate p-Toluolsulfonsäure monohydrate |
| Pronunciation | /ˈfɔːr təˈluːiːnˌsʌlˈfɒnɪk ˌæsɪd ˌmɒnəʊˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | “6192-52-5” |
| Beilstein Reference | 1720232 |
| ChEBI | CHEBI:39870 |
| ChEMBL | CHEMBL1376 |
| ChemSpider | 20259 |
| DrugBank | DB14004 |
| ECHA InfoCard | 03f6451f-d56a-4cae-bc77-0859d2d3fd94 |
| EC Number | 200-149-6 |
| Gmelin Reference | 82502 |
| KEGG | C02582 |
| MeSH | D013053 |
| PubChem CID | 23665780 |
| RTECS number | WN5600000 |
| UNII | PV6V8U214W |
| UN number | 2582 |
| Properties | |
| Chemical formula | C7H8O3S·H2O |
| Molar mass | 190.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.4 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.2 |
| Vapor pressure | 0.951 hPa (25 °C) |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | -6.5 |
| Magnetic susceptibility (χ) | -6.43e-6 cm³/mol |
| Refractive index (nD) | 1.594 |
| Dipole moment | 2.88 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 192 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -887.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1674 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P264, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338, P310, P321, P363 |
| NFPA 704 (fire diamond) | 3-0-1-Acidity |
| Flash point | 86 °C |
| Autoignition temperature | 180 °C |
| Lethal dose or concentration | LD50 Oral Rat: 2,480 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (Rat): 2480 mg/kg |
| NIOSH | WH3550000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Toluenesulfonic Acid Monohydrate: Not established |
| REL (Recommended) | 1 mg/m³ |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid Methanesulfonic acid 4-Toluenesulfonic acid 4-Toluenesulfonyl chloride o-Toluenesulfonic acid |