Chemistry classrooms always highlight the importance of some unsung workhorses, and 4-toluenesulfonic acid is a great example. Back in the early 1900s, German chemical industries valued this compound for its reliability in organic synthesis, quickly finding a spot in dyes and pharmaceuticals. Over time, labs worldwide picked up on its solid acid strength, favoring it over sulfuric acid in many reactions. Generations of chemists got their start learning about esterification and condensation reactions made easier by this particular acid. Anyone interested in industrial history will notice that its widespread use tracks the growth of aromatic chemistry and the rise of specialty chemicals for everything from detergents to electronics.
Most chemical suppliers sell 4-toluenesulfonic acid as a white crystalline powder, sometimes in a monohydrate form. Chemists count on its strong acidity, which stands out for working in both water and organic solvents. You can spot its clean, pungent odor even when handling it in small batches. Production facilities have standardized this item because decades of feedback from industrial and research chemists demand consistent, high-quality material. It doesn’t tend to clump or degrade in typical settings, so handling, storage, and weighing rarely cause trouble for operators who have spent years in practical labs.
Solid at room temperature, 4-toluenesulfonic acid melts just above 100°C and dissolves well in water, alcohol, and ethers. Its acid strength rivals hydrochloric and sulfuric acids but, thanks to its aromatic backbone, it offers greater stability for certain organic reactions. Its pKa hovers around -2.8, giving it plenty of punch for dehydration and catalysis. The molecule itself features a sulfonic acid group firmly attached to a methyl-substituted benzene ring, presenting both hydrophobic and hydrophilic facets that widen its scope across synthetic and industrial applications.
On a typical bottle's label, you’ll often find the minimum assay listed (often above 99%), melting range, water content (for the monohydrate), and the CAS number 104-15-4. Reputable suppliers track heavy metal content, giving buyers confidence for both pharmaceutical and food-grade work. Quality control labs measure color, acidity, and particle size, since these can affect handling and reactivity on a practical scale. Most packaging includes clear pictograms for safe handling, noting the usual irritant and corrosive hazards, which I always appreciate as someone who’s seen too many scarred gloves and benchtops.
Industry typically prepares 4-toluenesulfonic acid by sulfonating toluene with concentrated sulfuric acid, followed by careful crystallization and purification. Decades ago, these processes would churn out mixed isomers and significant waste, but improved catalysts and continuous-flow systems now raise yields while curbing environmental impact. Large plants reuse the spent acid stream, optimizing raw material use and lowering costs. In smaller labs, the procedure rarely changes: toluene, sulfuric acid, lots of stirring, and patience for crystal precipitation. Those long hours waiting for crystals gave me plenty of time to develop troubleshooting skills and a respect for quality glassware!
Practically every synthetic organic chemist has leaned on 4-toluenesulfonic acid at some point. Its strong acidity but low volatility make it a favorite for catalyzing esterification and alkylation reactions. In my own experience, converting alcohols to tosylates using the corresponding chloride (tosyl chloride) always seems cleaner and less finicky than other sulfonylations. 4-toluenesulfonic acid can also trigger dehydration of carbohydrates, initiate cationic polymerizations, and facilitate protecting group strategies. Thanks to its sturdy aromatic ring, it stands up to tough reaction conditions without introducing side products or unwanted color impurities.
You’ll often see 4-toluenesulfonic acid listed as p-toluenesulfonic acid or PTSA. Some catalogs call it para-toluenesulfonic acid, emphasizing the 4-position of the methyl group. In research literature, the acronym TsOH comes up frequently. Despite all the synonyms, a trained chemist can pick out the molecular formula C7H8O3S and know exactly what to expect in terms of performance and hazards.
Safety deserves steady focus no matter how familiar a compound may seem. 4-toluenesulfonic acid burns on contact and releases heat and fumes if mixed with bases or water too quickly. My early days in the lab taught me to respect the sharp bite of sulfonic acids and protect my hands, face, and bench from splashes. Detailed risk assessments insist on chemical goggles, gloves made from nitrile or butyl rubber, and a strong fume hood draw for weighing and mixing. On-site storage facilities keep it in sealed, labeled containers to limit accidental exposures. Well-written safety datasheets underline emergency procedures and disposal rules, which protect operators and maintenance crews from long-term health risks.
The reach of 4-toluenesulfonic acid spans diverse industries. In pharmaceuticals, it catalyzes complex syntheses with fewer by-products compared to mineral acids. Paints and plastics manufacturers appreciate its tough but selective acid action, ensuring color and clarity in resins and coatings. Water treatment companies sometimes rely on its strong acidity for specialty ion-exchange formulations. Electronics producers use it for etching and circuit patterning. Even in cosmetics, trace use helps adjust pH and support emulsion stability. No matter the trade, you’ll find process engineers who consider PTSA a vital ingredient for delivering reliable results batch after batch.
Universities and corporate labs never stop looking for cleaner or more efficient ways to catalyze key transformations, and 4-toluenesulfonic acid remains a benchmark. My time in academic labs often involved head-to-head tests of strong acids, and PTSA earned high marks for minimal side reactions and easy separation from products. Development teams keep refining its role in greener processes, for example, by pairing it with solid supports or ionic liquids. Experimentalists design new derivatives—some bulkier, some tricked out with fluorine or alkyl chains—to fine-tune solubility or reactivity for next-generation materials and pharmaceuticals.
The straightforward toxicity profile doesn’t mean this compound is benign. Rapid testing shows skin, eye, and respiratory irritation after contact or inhalation, which lines up with its strong acid nature. Animal studies point to low potential for chronic damage at modest exposures, yet industrial hygienists flag repeated respiratory and dermal contact as a real risk for workers. Toxicologists assess aquatic and environmental hazards, and regulatory agencies require strict documentation of waste discharge and emergency spills. Seeing the regulations tighten year by year reminds me that chemical safety evolves alongside new research and growing workforce awareness.
Demand for efficient, recyclable catalysts only climbs as the world leans into sustainable manufacturing. 4-toluenesulfonic acid has solid prospects, given its performance in green chemistry setups and process intensification. Researchers keep searching for ways to anchor it on reusable polymer beads or nanoparticles, aiming for zero-waste synthesis. Collaborative projects between universities and industry partners look at cleaner synthetic approaches, aiming to trim water and energy use. Across my career, fresh applications always follow new discoveries in catalysis and advanced materials, making 4-toluenesulfonic acid a steady fixture in the chemical innovation pipeline.
People rarely stop to think about what goes into making products last longer or work better. One name that pops up in chemical circles more often than you’d think is 4-toluenesulfonic acid. Known by chemists for its power to kickstart or speed up reactions, this compound shows up not just in labs but also in places that reach just about everyone.
In the world of plastics, getting small molecules to link up and form something useful takes skill. I remember the first time I visited a resin plant—safety goggles fogged up, the machinery humming—and saw 4-toluenesulfonic acid used right on the factory floor. The workers kept a close eye on the mixing tanks, making sure the acid acted as a strong acid catalyst to help shape tough, durable polymers. Its high solubility and strength mean it can push along reactions where weaker acids just fizzle out.
This matters in everyday life. Epoxy adhesives, coatings, and insulation owe their reliability in part to the acid doing its job behind the scenes. No one wants their kitchen counter to peel at the edge or their electronics to fail because a resin didn’t set up right. Quality control in manufacturing circles back to using the right catalyst, and 4-toluenesulfonic acid often fits the bill.
Pharmaceutical manufacturing is another lane where this acid shows up. Labs use it as a catalyst to string together complex molecules with intent—less time, fewer side products, purer drugs. That makes a difference in the real world, where a single impurity can put a patient at risk. When it comes to scaling up, reliability matters, and chemists trust 4-toluenesulfonic acid for repeatable results.
Think of pain relievers, antibiotics, or even vitamins. During manufacture, keeping reactions under control and ensuring maximum yield supports both safety and affordability for patients. At my previous job, we studied yield improvement for an over-the-counter drug, and switching to this acid saved both time and money in the process.
Beyond plastics and pills, 4-toluenesulfonic acid finds its way into detergents and dyes. Bright color in your clothes or fade-resistant shades on home décor go through manufacturing steps that often tap acidic catalysts. The acid strips away protective groups in dye chemistry, allowing vibrant colors to lock into textiles.
Even in dish soap or laundry detergent, the acid’s presence nudges chemical reactions to the finish line, helping make concentrated, reliable cleaners. This supports less waste, stronger cleaning power, and more sustainability in household products.
With all these benefits come some challenges. Working around a strong acid calls for better safety protocols. Protective gear and good ventilation are musts—nobody wants chemical burns or accidental exposure in the workplace. Safe storage and handling haven’t always kept up with smaller manufacturers, making oversight important.
There’s also an environmental footprint to consider. While 4-toluenesulfonic acid helps make things cleaner and longer-lasting, chemical waste and runoff can harm local waterways if disposal isn’t planned out. Some companies look to process intensification or green chemistry approaches, reducing waste or recycling catalysts whenever possible. Today’s push for tighter environmental rules means everyone in the supply chain needs to improve tracking and management.
The story of 4-toluenesulfonic acid serves as a window into how essential chemicals touch everyday life in ways most people never see. Smart management, safe practices, and ongoing innovation will help keep both workers and the environment safe without sacrificing product quality.
4-Toluenesulfonic acid, often known as p-toluenesulfonic acid or TsOH, pops up almost every time someone talks about organic chemistry labs or certain manufacturing processes. In the real world, it’s prized for its ability to catalyze reactions, especially when companies or researchers want to make chemicals in a cleaner, more efficient way than with mineral acids like sulfuric acid.
TsOH brings clear risks. Once exposed to the air, its white, crystalline form can draw in water, which means it sticks to skin more easily, increases the risk of burns, and can worsen damage if spilled. Breathing in the dust feels harsh, stings the nose and throat, and, according to the CDC, can provoke coughing, shortness of breath, or even more serious lung irritation after repeated exposure. A single careless splash on bare skin quickly becomes painful. Its corrosive nature leaves burns, redness, and swelling. Getting some in your eyes brings searing pain and runs the risk of lasting damage. Swallowing it causes burning from the mouth to the stomach, which never ends well.
Not enough gets said about what happens if TsOH escapes into waterways or soil. It dissolves quickly, but its acidity can kill aquatic life, wipe out bacteria that help plants grow, or shift groundwater pH. Companies are supposed to treat waste streams so acidic chemicals never reach rivers, but slip-ups happen. Minor spills on site usually get neutralized with basic compounds or swept up with absorbant material, but large leaks need emergency action to avoid environmental harm.
Regulators know how caustic TsOH acts. The Occupational Safety and Health Administration classifies it as hazardous. Workers must gear up with gloves, goggles, lab coats, or chemical suits in case of spills or splashes. Labs or factories must prepare safety data sheets, provide proper training, and have clear emergency plans. Regular inspections keep everyone honest, and penalties for shortcuts can bite.
People who have spent enough time in research or chemical manufacturing rarely forget their first TsOH accident. I watched a colleague in grad school fumble a beaker and wind up dousing her hand—gloves saved her from worse, but she still needed first aid and days to recover. Safety gear, neutralizer stations, and clear labeling don’t seem like luxuries after that. They’re the foundation of a healthy workplace.
Solving the hazard problem around TsOH hinges on three things: information, equipment, and habit. Training new workers in real, scenario-based situations sticks with people far longer than dry instructions. Stocking up on quality gloves, splash goggles, and fast-access showers goes further than cheap fixes or ignoring best practices. Finally, developing a real safety culture—where workers speak up about unsafe practices and actually pause to grab the right protective item—prevents mistakes from turning into medical emergencies.
Calling TsOH outright dangerous misses the point. Treated with respect, it helps research progress, keeps costs low, and avoids heavier pollution linked to stronger acids. When mismanaged, the consequences pile up fast. Over my years in chemistry, I’ve learned that clear procedures, tough oversight, and an honest look at environmental impacts shape the safest handling. No shortcut replaces simple respect for a hazardous chemical.
Anyone who’s spent time in a lab likely recognizes 4-Toluenesulfonic acid, known by the formula C7H8O3S. Most people see it written as TsOH or p-TsOH. This compound comes as a white, sometimes off-white solid. On the bench, it often shows up as either a crystalline powder or small, sugar-like granules. Get up close, and the sharp, slightly acrid odor is impossible to ignore. At ordinary room temperatures, nothing about its look suggests the strength it packs in chemical reactions.
4-Toluenesulfonic acid matters well beyond its formula. It works as a strong organic acid, up there with the mineral acids, but easier to store and handle than something like sulfuric acid. In my own work, grabbing a jar of p-TsOH always signals a day spent speeding up esterification or pushing a stubborn dehydration over the finish line. Unlike many mineral acids, p-TsOH dissolves easily in a range of organic solvents and water, which expands its usefulness. Small molecule synthesis would bog down without it. Even students balancing flasks in undergraduate labs lean on its reliable performance.
This acid’s solid, non-volatile nature stands out. Carrying or measuring it rarely leaves you worrying about fumes or nasty burns on your skin. That said, get it on your hands and you’ll feel the sting pretty quickly. Pair it with alcohols, and it yields neat toluenesulfonate esters — useful intermediates for swapping out functional groups, especially in pharmaceutical research. Companies making dyes and specialty chemicals rely on it for everything from sulfonation to resin prep.
Despite being easier to handle than a bottle of hydrochloric acid, p-TsOH’s dangers can’t be shrugged off. Inhaling dust triggers coughing and irritation fast. Spilling a heap on the bench? You might see it start to eat away at the surface if it sits too long. Safety glasses and gloves aren’t just lab fashion, they’re necessities. I’ve lost track of how often a rushed researcher wipes their eyes after measuring out toluenesulfonic acid, leading to a mad dash for the eyewash station.
Waste cleanup needs care. Pouring it in the sink leads to corroded pipes and real headaches for anyone maintaining the lab. Pouring old acid down the drain goes against every rule in waste management. Most safety protocols call for neutralizing strong acids before disposal. Many labs use sodium bicarbonate solutions, which brings the pH to a safe range, protecting both people and plumbing.
The responsible use of 4-Toluenesulfonic acid depends on clear procedures. Training newcomers on what spills and dust can do goes a long way in preventing serious incidents. In our lab, color-coded containers and careful labeling help prevent confusion, especially in crowded storage areas. Regular equipment cleaning, decent ventilation, and routine checks of storage shelves might sound boring, but these habits cut down on accidents involving strong acids.
For professional chemists and students alike, keeping an eye on safety data sheets and proper storage fights complacency — a key ingredient for safe, productive lab work. Modern chemistry wouldn’t move as quickly without compounds like 4-Toluenesulfonic acid, but respect for its hazards remains part of the scientific craft.
4-Toluenesulfonic acid, or PTSA as it often gets called in the lab, plays a big role as a catalyst and reagent for a bunch of chemical processes from making medicines to dyes. It’s a workhorse, but like any chemical that packs a punch, it needs real attention when brought into the workspace.
This acid isn’t fond of moisture and prefers places with steady, cool temperatures. Resealable containers work best—think thick plastic or glass with tightly fitted lids. Metal containers never go well with strong acids, which chew right through with time. Keep PTSA out of direct sunlight or anywhere humid. Humidity gets inside, and soon you’re dealing with clumping or even chemical reactions that launch a headache for any chemist.
Every time I visit a lab, I check the label and container seals before pouring or measuring. Expensive ingredients and ruined experiments have burned that habit into my routine. PTSA eats right through cheap or cracked containers, so don’t trust old, cloudy plastic. Shelving space matters, too. Only store it at eye level or lower, where spills are less likely to splash you or others. Never put acids near bases or solvents, since those separate properties lead to hazardous situations if a spill or leak happens.
Nothing beats hands-on reminders. Once, as a graduate student, I helped clean up after someone tossed PTSA into a sink. Fumes filled the lab, and gloves melted. Always wear protective gear: tight-fitting goggles, gloves made for chemicals (not just dish gloves), and a sturdy lab coat. Respirators should be on hand, especially if you don’t know how long you’ll be exposed or if larger volumes are in play.
Dust control matters. PTSA is a fine, crystalline powder, and open windows, running fans, or rough handling lift that dust into the air or onto skin. Use a scoop, avoid pouring straight from the main container, and work in a fume hood whenever possible. Even working with small amounts builds up exposure over time, so investing in proper equipment and protective barriers keeps problems small.
Accidents stick with you. I’ve watched colleagues freeze up faced with a spill. Instead, know your protocol ahead of time. Neutralize spills with sodium bicarbonate powder, keeping it ready at all times. For any skin or eye contact, flush with lots of water and seek medical attention. Don’t try to sweep up dry spills—dampened paper towels or dedicated acid spill kits keep dust down.
Leaks often point to storage mistakes or worn containers. Replace any questionable packaging right away and relabel each time. In shared workspaces, clear signage matters. Store the emergency numbers where you can read them fast, not locked in a drawer. These tricks save minutes that count—nobody forgets their worst day in the lab easily.
For all its power, PTSA invites a straightforward approach: tight lids, dry cool space, and personal protection at every turn. Labs should train everyone, not just the lead chemist. Posting checklists and keeping gear within arm’s reach removes guesswork and keeps small hiccups from growing. 4-Toluenesulfonic acid won’t give second chances, but care and a bit of old-fashioned caution keeps dramatic stories at bay.
Chemists know how much time can slip away hunting for good solvents. Take 4-Toluenesulfonic acid for example. This common acid pops up in everything from organic labs to pharmaceutical synthesis. Folks often need it as a catalyst, a reagent, or just to adjust pH. Some days, what matters most: will it actually dissolve? This acid doesn’t behave like table salt or sugar; it has its own set of rules that matter for both small and large-scale work.
Water treats 4-Toluenesulfonic acid like an old friend. Even at room temperature, it dissolves with little fuss. A lot of organics don’t play this way. Put a few grams of this acid in cold water and watch it vanish. So, water-based chemistries get a boost from this friendliness. Years in the lab taught me that polar solvents treat this acid with respect. Methanol, ethanol, and even acetone work well. You’ll see quick dissolving action, no heating or violence required.
Flip the coin. Try tossing it into standard non-polar organics like hexane or toluene. Good luck. It just sits there, clumping up and refusing to budge. You can stir all day; nothing good comes out of it. Even as the acid's structure looks aromatic, the sulfonic group drags strong polarity into play. That polarity acts like a passport—in there with water, ignored by oil. In my experience, solvents like chloroform give mixed results: some solubility, but nowhere near the numbers water brings. Diethyl ether doesn’t help much either.
Not every researcher or plant engineer cares about the fine print, but solubility dictates how fast, easy, and safe a reaction can run. Some acids like this can gum up gear, make pastes, or sit useless at the bottom of a flask if mixed in the wrong media. If you see cloudiness or chunks, time to pick another solvent.
Trying to use 4-Toluenesulfonic acid in ether or toluene isn’t just frustrating. It can slow a whole process, raise costs, and even bump up accident risks if crystals hang around and block filters. Water, methanol, and ethanol make cleanup smoother, and keep processes running without clogging up. In bigger reactors, solubility keeps pumps and pipes clear, and avoids losses during isolation.
Organic labs and drug makers chase after yield and purity. Using the right solvent affects both. For 4-Toluenesulfonic acid, sticking with water or alcohols makes the difference between a clean synthesis and a week of head-scratching. Even industry safety teams appreciate this; predictable solubility means fewer slip-ups. Folks have learned that changing temp or stirring harder doesn’t help if the rules of polarity aren’t being followed.
So what should someone do when water or methanol can’t be used? Buffered solutions or phase transfer catalysis sometimes open doors. Salts of the acid, like the sodium or potassium form, shift solubility and offer workarounds. But most days, starting with water or a short-chain alcohol avoids headaches.
Experience keeps teaching the same lesson: solubility rules shape outcomes more than reaction speed or fancy glassware. With 4-Toluenesulfonic acid, respecting polarity and solvent choice saves cash, time, and stress. It’s just one more example of how small details in chemistry often have some of the biggest impacts outside the spotlight.
| Names | |
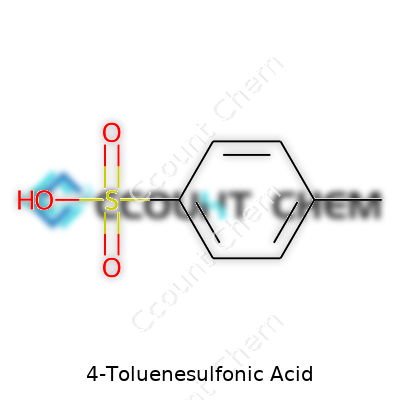
| Preferred IUPAC name | 4-methylbenzenesulfonic acid |
| Other names |
p-Toluenesulfonic acid PTSA p-Toluenesulphonic acid 4-Methylbenzenesulfonic acid Tosic acid Tosyl acid |
| Pronunciation | /ˈtɒl.juːiːn.sʌlˌfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 104-15-4 |
| Beilstein Reference | 636953 |
| ChEBI | CHEBI:28718 |
| ChEMBL | CHEMBL1402 |
| ChemSpider | 5461 |
| DrugBank | DB14260 |
| ECHA InfoCard | 100.015.398 |
| EC Number | 207-673-6 |
| Gmelin Reference | 82110 |
| KEGG | C01400 |
| MeSH | D014027 |
| PubChem CID | 6112 |
| RTECS number | WN5600000 |
| UNII | R1XW24Q5GL |
| UN number | UN2583 |
| Properties | |
| Chemical formula | C7H8O3S |
| Molar mass | 190.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.24 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.6 |
| Vapor pressure | 0.05 mmHg (25°C) |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | -6.5 |
| Magnetic susceptibility (χ) | -70.9e-6 cm³/mol |
| Refractive index (nD) | 1.587 |
| Viscosity | 5 cP (20°C) |
| Dipole moment | 1.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 203.3 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -796.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2046 kJ mol⁻¹ |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H290, H314 |
| Precautionary statements | P264, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 3-0-2-A |
| Flash point | > 107°C |
| Autoignition temperature | 480 °C (896 °F; 753 K) |
| Lethal dose or concentration | LD50 (oral, rat): 2480 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Toluenesulfonic Acid: Oral rat LD50 = 2480 mg/kg |
| NIOSH | WGK 1 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL: 5 mg/m³ |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid Methanesulfonic acid Toluene p-Toluenesulfonyl chloride o-Toluenesulfonic acid Sulfanilic acid |