Chemistry keeps moving fast, but for folks working with aromatic compounds, 4-Nitro Aniline-2 Sulfonic Acid holds a steady place on the workbench. Researchers and industry professionals started paying attention to this compound in the early twentieth century—around the time dyestuff chemistry boomed and colorants grew important in everything from textiles to plastics. Chemists saw the sulfonic acid group as a tool that let dyes grip fibers better, and with the nitro and amino groups already popular for color development, it didn’t take long for the combination of all three to turn 4-Nitro Aniline-2 Sulfonic Acid into a key piece in many synthetic routes. Looking through the old literature, there’s evidence that pioneers in European chemical companies explored this molecule to improve print clarity and shade fastness, which still matters very much to end users today.
Today, the compound shows up most in the form of a yellow or light brown crystalline powder, usually packed in lined fiber drums or HDPE bags. Commercial production aims for a fine consistency, so the material dissolves smoothly when formulating dyes, pigments, or specialty chemicals. Large-volume orders, often topping a few metric tons, roll out to textile processors, ink manufacturers, and sometimes pharmaceutical intermediates suppliers. Each batch comes with a dedicated lot number for tracking, as most regulatory standards push for transparency in chemical origin and processing chain.
4-Nitro Aniline-2 Sulfonic Acid lays out its complexity in a surprisingly straightforward way for anyone who’s handled aromatic compounds. Its melting point falls on the low side, between 240°C and 250°C, and the molecule stays stable under normal storage, but high humidity introduces risk—lumps in the powder mean trouble mixing later on. The structure features both electron-withdrawing nitro and sulfonic acid groups, with an amino group providing a balancing act that affects solubility and reactivity. Water solubility climbs as pH shifts into alkaline territory, a property that suits textile dye baths. The acid stands up well to mild oxidizers but reacts quickly with alkalis and strong reducing agents. For people working in the lab or on the plant floor, the strong, somewhat pungent smell makes its presence known, so good ventilation counts for a lot.
A reliable supplier will always give you more than just a purity claim. Quality standards usually demand an assay above 96%, with chlorine, insoluble matter, and heavy metal content kept well below strict ppm levels. The pH of a 10% aqueous solution tends toward acidic (often around 1.5 to 2.5). Tech sheets lay out colorimetric or titrimetric purity checks and UV-vis data, always backed by batch-specific Certificates of Analysis. Labels on containers note full chemical names, synonyms, net weight, CAS number (which remains the go-to cross-reference for procurement teams), hazard pictograms, storage advice, and detailed handling precautions. Reliable labeling supports smooth movement through customs and downstream distribution.
Older texts and modern plants both stick with classic aromatic sulfonation, usually starting from 4-nitroaniline. Commercial synthesis involves adding fuming sulfuric acid or oleum to cooled nitroaniline, keeping temperatures under 25°C to manage exothermic heat. The reaction mass gets diluted, neutralized, and filtered; crude product goes through further purification by recrystallization—often using salt solutions to control particle size and purity. Energy efficiency matters a lot now, so process engineers experiment with heat recovery and optimized mixing to shave off costs. Waste stream management consumes equal attention: spent acids must be neutralized and sometimes processed for recovery of sulfur compounds.
4-Nitro Aniline-2 Sulfonic Acid earns its spot as a workhorse intermediate thanks to its reactivity profile. The amino and nitro groups participate in reduction, acylation, and diazotization, while the sulfonic acid function supports salt formation or coupling with metal ions. Dyes for cotton often start with diazotization of the amino group, yielding key intermediates for azo linkage. Reducing the nitro group provides access to diamino derivatives, which branch into pharmaceutical building blocks and complex colorants. Sulfonic acid itself endures tough processing steps, resisting hydrolysis and oxidation. Given its versatile structure, researchers keep finding new tweaks that lead to specialized colorants, ion exchange resins, and sometimes specialty pharmaceutical scaffolds.
Expect to see a handful of aliases, depending on which catalog you pick up or which continent you’re ordering from. Some call it “4-nitro-2-aminobenzenesulfonic acid” or “2-amino-5-nitrobenzenesulfonic acid.” Old industry literature and European suppliers might tag it as “2-sulfamino-4-nitrobenzene” as well. Commercial mixtures for specific dye preparations sometimes use trade names that mask the precise proportions of isomers—worth remembering if product quality drifts from batch to batch.
Anyone running a plant, lab, or warehouse knows safety matters more than any theoretical yield. 4-Nitro Aniline-2 Sulfonic Acid brings moderate health risks when inhaled or in contact with skin. Nuisance dust becomes an issue fast if ventilation isn’t there. Basic rule: gloves, goggles, dust mask, and—when mixing or transferring—mechanical ventilation at every station. Sets of onsite MSDS documents should outline risks for methemoglobinemia, eye and respiratory irritation, and fire-fighting procedures. Spillage goes straight to chemical spill kits, with waste streams labeled for manifesting. Employee training now covers not just acute toxicity but long-term exposure checks, as nitroaromatic compounds demand elevated scrutiny under labor safety laws.
Anyone walking the floor of a textile dye house, inkjet ink operation, or chelating agent plant will see the value right away. Most production targets go straight into azo dyes for cellulosic fibers—think cottons and rayons—because the sulfonic acid moiety locks color without shrinking wash fastness. Paper processing names this compound in specialty toner formulations, with pigment manufacturers using the acidic group to anchor binding agents. Beyond dyes, researchers working in advanced polymer electrolytes turn to it for both functionalization and ionic conductivity improvements. Specialty pharmaceutical syntheses apply the tinctorial and reactivity benefits when scaffolding harder-to-make intermediates. This wide reach sets it apart from more narrowly focused aromatic compounds that serve only one niche.
University and company labs keep looking for cleaner, faster, safer ways to make and use 4-Nitro Aniline-2 Sulfonic Acid. Green chemistry initiatives push for mild sulfonation in ionic liquids or deep eutectic solvents, which promise lower waste streams and smaller energy footprints. Catalytic strategies for nitro reduction continue to cut down on metallic waste. Ongoing work in fiber-reactive and acid dye families positions this compound as a foundation for more vivid, longer-lasting colors. Analytical chemists publish new quantitation and identification methods, supporting trace detection in environmental monitoring and product quality checks. Each year, more patent filings mention this acid’s role as a customizable intermediate, which signals there’s still room for technical improvement.
Toxicologists and regulatory bodies share real concern over nitroaromatic exposure in the workplace. Animal studies show methemoglobinemia as an acute risk, with chronic exposure risks including damage to blood and liver. Environmental chemists flag persistence of sulfonic acids, which can build up downstream if wastewater treatment skips some steps. Safety protocols grow stricter every year, with workplace air and water effluent thresholds running tighter all the time. Emerging analytical techniques support better monitoring in busy production environments, and enforcement agencies now hold plants to clear, actionable records on product handling, storage, and disposal. This contributes to worker safety and environmental stewardship in both developing and mature markets, creating a baseline for continuous improvement across industries.
People who follow the future of specialty chemicals see big changes ahead. Sustainability pressures push synthetic chemists to design cleaner routes and improve recoverability. Energy-smart plants seek value from process optimization, pushing for heat integration and real-time monitoring along the line. Academia and industry both show growing interest in cross-disciplinary applications, like advanced battery electrolytes or smart pigments that change shade under specific conditions. Additive manufacturing and digital printing demand novel dyes and pigments, with researchers looking at modified sulfonic acids for increased compatibility and performance. The next decade could bring major shifts, as regulatory and market trends push for lower-impact, higher-value products rooted in foundational compounds like 4-Nitro Aniline-2 Sulfonic Acid.
Step inside any textile factory and you hear the hum of machines pumping out fabric in every color. Those bright reds, oranges, and even some darker shades have a complicated lineage. 4-Nitro Aniline-2 Sulfonic Acid plays a pivotal part in synthesizing dyes that snap with color. Walk down the aisle in a clothing store, and you brush past the work of this compound. It’s a go-to building block, especially where dyestuffs for cotton or wool are needed.
Its role starts with the way it brings a nitro group and a sulfonic acid group together on a single aromatic ring. This setup allows the molecule to react well with other ingredients in the dye-making process, letting manufacturers tweak colors and water solubility. After years working with chemical suppliers, I’ve seen first-hand how suppliers lean on compounds like this for stable, reliable shades. Factories want ingredients that resist fading from washing or sunlight, and this acid fits the bill.
Dyes based on this molecule often stick better to fabrics and hold up after multiple washes. Brands can produce clothes that last longer and hold onto their color even with heavy use. In India, where textile processing is a major industry, sustainability pressures have grown. The ability to create durable dyes keeps waste down since clothes remain usable longer, reducing how often shoppers replace their wardrobe.
Factories also avoid certain heavy metals and other toxic agents by relying on this acid. Production lines can sidestep harsher chemicals, cutting back on environmental strain. Researchers have published studies—like those from the Journal of Applied Polymer Science—showing the compound’s efficiency in dye bonding, which is great news for manufacturers faced with tough environmental oversight.
Look outside textiles and you see 4-Nitro Aniline-2 Sulfonic Acid lending a hand in specialty pigment production. Some printing inks and coated papers rely on its presence, especially in markets demanding sharp, long-lasting hues. Last year at an expo in Mumbai, industry specialists highlighted how the chemical fills a niche between conventional dye precursors and specialty pigments, giving suppliers more ways to fine-tune their products for everything from packaging to industrial labeling.
Handling any nitroaromatic compound needs care. The chemical structure can pose risks if employees don’t respect the safety data sheets. Protective equipment isn’t optional in these manufacturing spaces because compounds like this can cause health complications with enough exposure. Countries like Germany keep a close regulatory eye on this family of molecules. I’ve sat through enough training on chemical handling to know mistakes here aren’t cheap.
With environmental groups spotlighting chemical runoff, many big players in the industry install advanced filtration. Some look at emerging techniques to treat effluents and capture or neutralize leftover compounds. This approach isn’t just about penalties—brand reputations hinge on how responsibly businesses treat water sources and local wildlife.
The tug-of-war between cost, performance, and environmental impact never slows. Continuing research into greener synthesis routes could lower the environmental load. Open dialogue among manufacturers, environmental scientists, and regulators matters. As demand for varied, high-quality dyes keeps rising, finding smarter ways to use and dispose of 4-Nitro Aniline-2 Sulfonic Acid stands at the center of responsible industry practice.
Anyone who remembers hours spent in a school lab knows that every molecule tells a story. 4-Nitro Aniline-2 Sulfonic Acid stands out to chemists and industries that value precision because its structure reveals more than a list of elements. A little background: this compound builds on an aniline core, dressed up with nitro and sulfonic acid groups at specific positions. The chemical formula comes together as C6H6N2O5S. You get six carbons, six hydrogens, two nitrogens, five oxygens, and a single sulfur atom packed into every molecule.
Let’s talk placement. The nitro group lands at the fourth carbon, the sulfonic acid at the second, and the amino group marks the aniline backbone. These positions impact everything from color in dyes to reactivity in synthesis. Researchers and manufacturers study this arrangement to tweak physical properties, adjust reactivity, and control the outcome of chemical reactions. Even a single misplaced atom can send a synthesis off track.
I remember an undergraduate project where the absence of proper molecular identification set back my entire synthesis scheme. Analytical methods, from NMR to mass spectrometry, go hand-in-hand with structures like this to double-check what’s actually present. It’s not only theory—real-world results depend on precision at the molecular level.
Calculating the molecular weight means totaling up the atomic weights of every atom in the formula. For C6H6N2O5S:
Total molecular weight holds at 218.21 grams per mole. Such accuracy isn’t just for paperwork. In process chemistry, accurate weighing leads to reproducible batches, which is essential when scaling up.
In practice, 4-Nitro Aniline-2 Sulfonic Acid works as a key intermediate in the dye, pharmaceutical, and agrochemical sectors. Its fine-tuned structure controls both color properties and interactions with other chemicals. For example, textile companies rely on consistent quality—no one wants a batch of fabric to shift hues halfway through a run. The pharmaceutical space checks every intermediate’s specs for purity to ensure safety downstream.
Chemical safety and regulatory records, like those listed on PubChem and Sigma-Aldrich, show that both the formula and the molecular weight stay front and center. They help labs calculate safe storage limits and predict environmental behavior. Mistakes at this point waste resources or, worse, create safety hazards.
This molecule’s journey from lab bench to industry line highlights bigger points about chemical accuracy. Mistakes in formula or molecular weight calculations create ripple effects—from failed syntheses to mislabeling on shipment documents. Open databases and peer review in journals give labs a way to cross-check work. More robust digital tools and easier access to standards can strengthen reliability, especially for smaller operations without big budgets.
Chemists know there’s no shortcut around molecular certainty. With C6H6N2O5S and its 218.21-gram-per-mole fingerprint, both research and production get a fighting chance at success. Reliable facts build confidence, whether for a student or a manufacturing plant manager. Trust in chemistry starts with getting the basics right.
Anyone working with chemicals such as 4-Nitro Aniline-2 Sulfonic Acid understands that a simple oversight can turn into an expensive, even dangerous, situation. Years spent around labs have taught me that common sense plus a bit of respect for these compounds always beats overconfidence. Some of my colleagues took shortcuts only to end up with ruined batches or worse—calls to the safety officer. Handling this compound shouldn’t feel intimidating, but the hazards are real. Skin and eye irritation, strong reactions with incompatible materials, and tricky cleanup—these aren’t rare events seen only in textbooks.
The best defense against accidents begins the moment the drum or bottle arrives. A cool, dry, well-ventilated storeroom offers a buffer against both humidity and excess heat. Direct sunlight and fluctuating room temperatures chip away at chemical stability. Left in a humid spot, this compound tends to clump and degrade, which throws off lab results and impacts downstream applications. So, by keeping things dry and cool, you don’t just avoid emergency eyewash drills—you save your data from preventable errors.
4-Nitro Aniline-2 Sulfonic Acid reacts unfavorably with oxidizers, reducing agents, and certain bases. Regular inventory checks help catch leaks, corrosion, or contaminated bottles early. Labels resist smudging, contain hazard symbols, and list purchase dates so no one’s working with a container past its prime. Shelving should hold its weight and not crowd bottles together—over the years, I’ve seen crumpled shelves and toppled bottles from overloaded storage, creating chaos that could’ve been avoided with better organization.
Goggles, gloves, and lab coats don’t just make you look the part—they shield against splashes, dust, and accidental skin contact. The yellow tinge of this material makes spills easy to spot, but residues on tabletops hide until a careless arm picks them up. Washing hands before and after handling should become automatic as buckling a seatbelt.
Good ventilation—such as a chemical fume hood—prevents inhalation of fine dust. Small spills often tempt people to sweep them away, but wet wiping with plenty of water works better and keeps particles out of the air. Any mixing must happen slowly, using tools that won’t trigger unwanted chemical reactions. Never mix waste streams; a single splash can generate heat or release toxic gases fast. Years spent troubleshooting mishaps taught me to respect those warning signs on the label.
Clear, practical SOPs (standard operating procedures) should sit close by, not just in a forgotten binder. These written steps keep everyone on the same page. Lab managers earn their stripes checking in with staff, reviewing logs, and running drills as routine, not after the fact. Emergency showers and eyewash stations belong within a short dash from the workbench. Even with top habits, accidents slip through—improvised response wastes precious seconds. Fire extinguishers, spill kits, and first aid packs should have spots that everyone can find even with their eyes closed.
Disposal deserves as much attention as anything else. Disposing of 4-Nitro Aniline-2 Sulfonic Acid down regular drains can pollute water or create unsafe downstream reactions. Working with reputable chemical waste processors costs extra time and money, but it preserves reputations and keeps communities safe. Periodic training refreshes habits. Seeing mistakes corrected in real time builds a culture where good handling is second nature. In the end, a clear focus on keeping chemicals dry, secure, and well-documented pays off—not just in safety, but in smoother workflows and trusted results.
Ask anyone who's handled dyes or worked around fine chemicals: you start to respect the power and dangers these substances carry. 4-Nitro Aniline-2 Sulfonic Acid, often used in making pigments, dyes, and pharmaceuticals, is no exception. Many people overlook risk, trusting lab coats and gloves will do the job every time. But this acid poses specific hazards.
I've worked in labs where material safety data sheets take up an entire filing cabinet. You get used to scanning hazard symbols—but the facts behind those icons mean something real. Skin contact with nitro compounds can cause irritation or even burns. Inhalation may lead to headaches and breathing problems. Spills aren’t rare, and every cleanup teaches the body reacts quicker than you’d expect.
Exposure doesn't only happen at a bench. Powder dust can sneak through vents, settle on surfaces, and move beyond the confines of glove boxes. According to the National Institute for Occupational Safety and Health (NIOSH), nitroanilines—like the one in question—may impact liver and kidney function if exposure goes unchecked. Animal studies point to these risks, and occupational health data supports what many workers feel after repeated low-level contact: symptoms like fatigue or nausea can signal more insidious effects.
Acute poisoning doesn’t usually come from a single splash. Chronic exposure, the kind found in places where safety gets overlooked or proper air filtration falls behind, builds up over weeks or months. If you're working without the right masks or fume hoods, chances rise for adverse health events. I’ve seen colleagues develop allergies to common solvents and reagents, which starts as minor irritation and sometimes escalates to more serious sensitivities.
Getting safety right takes vigilance and updated knowledge. Gloves don’t last forever; goggles fog up but keep your vision safe. Handling 4-Nitro Aniline-2 Sulfonic Acid calls for chemical-resistant gloves, lab coats, and reliable eye protection. Regular training helps catch bad habits and reinforces correct decontamination techniques after spills or splashes.
Proper chemical storage limits accidental exposure. Closed containers and sturdy shelving make a difference. Good housekeeping habits—like immediate cleanup and clear labeling—can't be exaggerated. Factory layouts matter: separating chemical handling areas from common spaces reduces transfer through shoes or clothing.
People sometimes forget chemical hazards move beyond plant boundaries. Wastewater treatment remains critical. Discharges carrying nitro compounds threaten aquatic life even at low concentrations. Monitoring by independent labs helps protect both workers and the greater community. I’ve seen neighborhoods downstream from old dye plants struggle with lingering contamination decades after closure.
Health and safety regulations reflect real risk, not just paper requirements. Knowledge, habits, and respect for what these chemicals can do matter more than any single written rule. The smallest lapses in judgment can have consequences for people, pets, and the environment. The best defense relies on transparency, good training, and updated safety management, keeping every worker and neighbor protected from 4-Nitro Aniline-2 Sulfonic Acid’s toxic potential.
4-Nitro Aniline-2 Sulfonic Acid grabs attention with a bright yellow to orange crystalline powder. Anyone who’s handled chemicals in a lab can spot certain compounds based on color alone, and this one doesn’t just blend in with the rest of the shelves. I remember opening a tub in my university’s organic chemistry lab and being struck by the vibrant hue, a stark contrast against the sea of white powders. That color comes from the nitro group sitting on the benzene ring — a strong sign you’re not dealing with anything bland. The color intensity makes spills easier to detect and clean, which does help with safety, especially among less experienced lab techs.
This powder isn’t greasy, nor is it particularly clumpy, but it can generate some fine dust if handled carelessly, which ought to serve as a reminder about good ventilation and wearing a proper mask. The granules themselves are not sticky, and kick up small clouds if poured too quickly, leading to sore throats and coughs if you skip the fume hood. Given the compound’s connections to dye manufacturing, this punchy color also flags the risk of stains — highly visible and not particularly easy to scrub off your bench coat.
Some compounds like this give a straight answer about their behavior in water. 4-Nitro Aniline-2 Sulfonic Acid dissolves fairly well in water, especially warm or hot. The sulfonic acid group helps this out; it’s polar, draws water molecules, and drops the powder into solution easier than many other aromatic compounds. In a cool lab, stirring saves time and avoids stubborn residue on the bottom of your beaker. Folks in the dye and pigment industries benefit from this good solubility, since it streamlines formulation and cuts down on mixing time.
The nitro group on the aromatic ring works against solubility in some organic solvents, especially the non-polar ones. Try tossing this powder into acetone, ether, or toluene, and it sets up camp at the bottom. Solubility in strong mineral acids and alkaline solutions trends high, not much of a surprise for a sulfonic acid derivative. In everyday use, that means it can be blended into aqueous dye baths or used for further synthesis in acidic or basic water-based reactions, not in organic-dominated set-ups.
For folks scaling up in a plant or tackling lab-scale projects, efficiency hinges on a solid handle of solvent compatibility. Water’s low cost and safety profile help operators dodge some headaches, but it’s best not to rely on wishful thinking for tough dissolving jobs. Companies cut unnecessary expense by matching intended use with this solubility information, and avoid wasted raw material from failed dissolutions.
Recognizing the distinct color and form lowers the risk of handling errors. In settings where cross-contamination could spell lost batches or ruined experiments, that distinctive orange-yellow powder serves as a helpful red flag. Reliable solubility in water unlocks broader applications, from dyes to pharmaceuticals — anywhere a strong color and easy blending with water-based systems matter.
Lab managers who build up clear safety protocols around these properties create smoother operations and happier teams. The appearance of 4-Nitro Aniline-2 Sulfonic Acid signals caution, but not alarm — good labeling and proper PPE go a long way. Solid knowledge about water solubility speeds up training and stops confusion over why a reaction worked in one solvent and failed in another.
Looking ahead, wider adoption in sustainable dye processes and advanced pharmaceutical syntheses will always rely on this balance: bright, characteristic color and plain, no-nonsense water solubility. Efficiency and safety improve when labs and plants work with real facts and treat these materials with the respect built from both chemistry textbooks and practical, lived experience.
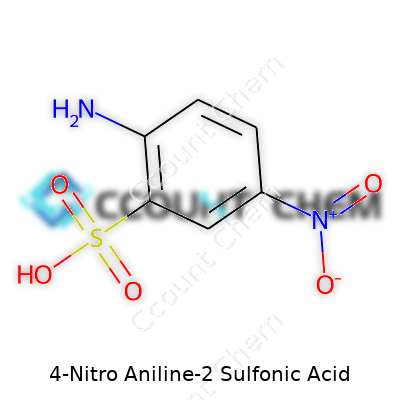
| Names | |
| Preferred IUPAC name | 4-nitrobenzene-1,2-diamine-1-sulfonic acid |
| Other names |
2-Amino-5-nitrobenzenesulfonic acid 4-Nitro-2-aminobenzenesulfonic acid 4-Nitro-o-anisidine sulfonic acid |
| Pronunciation | /ˈfɔːr ˈnaɪtrəʊ əˈnɪliːn tuː sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 121-87-9 |
| 3D model (JSmol) | `/data/mol/chem/ID12/JSmol/4-Nitro_Aniline-2_Sulfonic_Acid.mol` |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:91216 |
| ChEMBL | CHEMBL105011 |
| ChemSpider | 184693 |
| DrugBank | DB14154 |
| ECHA InfoCard | ECHA InfoCard: 100.011.716 |
| EC Number | 258-961-6 |
| Gmelin Reference | 90346 |
| KEGG | C19270 |
| MeSH | D009638 |
| PubChem CID | 159912 |
| RTECS number | GN8575000 |
| UNII | 2CS768U41Q |
| UN number | UN number is "UN2662 |
| CompTox Dashboard (EPA) | DJ188922 |
| Properties | |
| Chemical formula | C6H7N2O5S |
| Molar mass | 232.19 g/mol |
| Appearance | Light yellow to brown crystalline powder |
| Odor | Odorless |
| Density | 1.59 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.4 |
| Vapor pressure | 0.0000165 mmHg (25°C) |
| Acidity (pKa) | pKa = 1.0 |
| Basicity (pKb) | 6.64 |
| Magnetic susceptibility (χ) | -77.0·10⁻⁶ cm³/mol |
| Dipole moment | 3.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of 4-Nitro Aniline-2 Sulfonic Acid is 319.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1446 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P305+P351+P338, P310, P501 |
| NFPA 704 (fire diamond) | 3-2-2 |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2820 mg/kg |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Nitro Aniline-2 Sulfonic Acid: 0.1 mg/m³ (inhalable fraction and vapor, as aniline) |
| REL (Recommended) | The REL (Recommended Exposure Limit) for 4-Nitro Aniline-2 Sulfonic Acid is 0.1 mg/m³ (as aniline, skin) as a time-weighted average (TWA) for up to a 10-hour workday. |
| Related compounds | |
| Related compounds |
Aniline 4-Nitroaniline Sulfanilic acid 4-Aminobenzenesulfonic acid 2-Nitroaniline 4-Nitrobenzenesulfonic acid |