4-Methoxy-Aniline-2-Sulfonic Acid has long played a silent, yet central, part in the broader chemical story since the mid-20th century. Organic chemists across Europe first described its production, mostly from the need to create complex dyes with strong colorfastness for textiles. In early records, technicians struggled with purity and yield, driving innovation in sulfonation and methoxylation. Early patents filed by German dye firms in the 1960s set the foundation for current practice. Over time, economic demand for better intermediates forced manufacturers to refine their extraction, isolation, and control of byproducts. Today’s methods stand on backs of these decades of small victories, showing just how crucial historical experience remains for materials that feed downstream industries.
4-Methoxy-Aniline-2-Sulfonic Acid lands squarely in the field of aromatic amines, standing out for how it combines a methoxy group in the para position with a sulfonic acid group at the ortho position. This dual functionality builds unique reactivity, helping it earn a spot as a reliable intermediate. Major pigment and dye makers treat this compound as a keystone, not just for its structure, but for its performance in acid dye syntheses. In day-to-day settings, technicians and researchers value its stability, solubility, and availability, seeing real-world benefits in batch reproducibility and fewer impurities in finished dyes.
4-Methoxy-Aniline-2-Sulfonic Acid usually appears as pale, tan crystalline powder or fine granules, sensitive to moisture and prone to caking without sealed storage. Its molecular formula, C7H9NO4S, points to a moderately compact structure. Water solubility runs high, thanks to the sulfonic acid function. The melting point ranges from 225°C to 230°C, which chemists need to track during both handling and scale-up. Odor remains faint, often described as mildly aromatic, much less pronounced than unsubstituted anilines. Adding the methoxy decreases electron density, reducing the nucleophilicity of the amino group just enough to open new options for selective substitution, while retaining enough reactivity for practical synthesis.
Specifications often require purity above 98% for critical applications. Chloride and heavy metal contents stay monitored, mainly due to their effect on dye purity and safety. Color index and residue on ignition set reference points for batch quality, especially where regulatory compliance matters. Packaging standards call for lined drums or sealed polybags to guard against moisture. On every label, UN numbers and hazard pictograms follow global safety standards, not only for customs compliance but for in-plant safety. Manufacturers stamp clear batch codes for traceability. Technicians routinely check that containers display correct gross weight, storage guidance, and expiry dates so every shipment aligns with operational and environmental rules.
Industrial processes most often begin with p-anisidine as the starting point, subjecting it to controlled sulfonation with fuming sulfuric acid (oleum) at precisely maintained temperatures. Skilled operators watch for exotherms, adjusting rate and mixing to curb runaway reactions. Isolation typically uses stepwise neutralization, filtration, and re-crystallization to reach target purity. Side products, like multi-sulfonated variants, get separated through careful pH control and solvent selection. Decades of process refinement cut waste output and water use, important for both efficiency and environmental compliance. Small labs favor direct batch synthesis, but large-scale plants run continuous setups with real-time monitoring to push yield, lower costs, and improve product uniformity.
Modification possibilities start with diazotization of the aniline group to launch azo coupling, creating strong, stable azo dyes. The methoxy part acts as both a mild electron donor and as a control point for further substitutions without triggering excessive side reactions. Chemists often use this acid in amide bond formation, especially during the creation of pharmaceutical intermediates. Under reducing conditions, deamination opens pathways to other aromatic structures, feeding both natural product synthesis and materials development. Custom derivatization with alkyl or acyl groups expands its catalogue, while cross-coupling reactions blend it into larger frameworks for molecular electronics or sensors.
Many names have gathered around 4-Methoxy-Aniline-2-Sulfonic Acid. In industry circles, it’s known as p-Anisidine-2-Sulfonic Acid, 2-Sulfo-4-Methoxyaniline, or Fast Red RC Base Acid. Different suppliers and catalogues highlight one name over another, depending on their sales regions and legacy customers. Large dye groups use brand names linked to old patents, which sometimes adds confusion for importers and new users. Sorting out synonym use proves crucial in both quality control and regulatory submissions. Chemical databases, like ChemSpider and PubChem, help bridge gaps where legal or compliance paperwork demands exact matches.
Safety rules matter here. Workers need gloves, goggles, and solid ventilation whenever handling powders in open vessels or transfer steps. Like many aromatic amines, 4-Methoxy-Aniline-2-Sulfonic Acid poses some health risk with long-term contact, especially through skin or inhalation. Regulatory guides, including REACH and OSHA, require training for proper spill response, disposal, and first aid. Waste streams get monitored for residuals that can stress local water systems. Regular air and surface monitoring in production zones helps catch leaks before they pose problems, and emergency shutdown drills stay a non-negotiable part of any operator routine.
Dye and pigment producers stand among the largest users of 4-Methoxy-Aniline-2-Sulfonic Acid, as it shapes both color tone and binding in finished textiles. Its dependable sulfonic acid group anchors dyes onto fibers, especially where wash fastness rates as critical. Research chemists value it for synthesizing new classes of chromophores, which can boost brightness or alter fluorescence for imaging systems. Beyond colorants, some pharmaceutical companies use it as an intermediate for advanced molecules, where both the amino and methoxy groups set up selective transformations. In printed circuit board manufacturing, new developments harness its electrophilic functions to fine-tune adhesive layers. Specialty polymer groups experiment with its incorporation for unique crosslinked structures, especially in filtration and ion-exchange materials.
Academic and industrial labs both keep pushing boundaries with this compound. Recent studies track greener sulfonation chemistry, swapping harsh acids for solid acid catalysts, shrinking waste. Teams in specialty dyes race to extend the shade and lightfastness by tweaking substituents around the aromatic ring. Pharmaceutical research watches for new derivatives that bring anti-inflammatory, antifungal, or anticancer properties. Process engineers run kinetic studies to cut batch cycle times, important where energy and labor costs push budgets. Sustainable process development, like closed-loop water systems and zero-discharge plants, climbs the priority ladder as both regulation tightens and customers look for smaller supply chain footprints. New hybrid materials using this acid attract attention among sensor companies, especially in wearable tech or confined-space diagnostics.
Toxicity reviews flag low-level risks for skin and eye irritation, especially for workers with repeated exposures. Early animal studies show that, while less hazardous than unsubstituted aniline or some sulfonated cousins, the compound needs respect in handling and disposal. Chronic low-dose studies in rodents point to possible liver or kidney effects, but no strong evidence has yet placed this acid among known carcinogens. Regulators call for further study on long-term environmental persistence, as its breakdown in water can lead to biologically active fragments. Companies invest in safer process lines, disposable gear, and exhaust filters to prevent accidental exposure. Waste management programs, relying on activated carbon and advanced oxidation, shrink the risk of downstream contamination.
As regulations clamp down on hazardous dye intermediates, the market for cleaner, more selective sulfonated aromatics looks strong. Advances in catalysis hint at cheaper, lower-impact chemical routes that may lower energy demand and reduce secondary pollution. Functional materials based on this acid could change how companies build filters or membranes for battery and water purification sectors. Pharma and medtech groups watch for new therapeutic candidates from derivatives, betting on both traditional and AI-driven discovery tools. With renewable chemistry on the rise, tomorrow’s plants may draw their feedstocks from biomass, cutting dependence on legacy petrochemicals. Safety standards will tighten, and old factories may face retrofits, but the compound’s versatility and proven value ensure it keeps a respected spot in the toolbox of scientists, engineers, and technologists worldwide.
Growing up around textile mills gave me a window into the chemicals that shape what we wear. 4-Methoxy-Aniline-2-Sulfonic Acid stands out as a key piece in this world. Chemists reach for it when mixing up special dyes, especially azo dyes. It pulls its weight by helping attach color to fibers in fabrics, making reds punch through instead of fading to a tired pink after one trip through the wash. The textile trade depends on it to make color fast and vivid, which keeps buyers and designers happy. It's not just for rich colors; it keeps those hues locked in through sunlight, sweat, and wear.
Printing wouldn't be nearly as sharp or colorful without this compound. Inks that mark newspaper pages and glossy magazines get their backbone from intermediates like 4-Methoxy-Aniline-2-Sulfonic Acid. Makers of printing inks say its presence punches up both the brightness and the staying power of their products. For any publisher or packaging plant, reliable ink is essential for both legibility and impact. Without sturdy colorants, prints would smudge or fade, leading to unreadable text and wasted paper.
Lab workers in medicine have long hunted for reliable building blocks, and this molecule clocks plenty of time on their shelves. Its sulfonic acid group opens pathways to drugs that demand both water solubility and reactive punch. Antibiotic and antihypertensive drugs can trace some of their roots to reactions built on this aromatic ring. The science cuts through theoretical chatter, since better solubility means smaller pills and doses that work more efficiently. Families rely on those breakthroughs. Reliable intermediates, like this one, speed up research, cutting down the time it takes to move from idea to treatment.
Tires and shoe soles look plain enough, but the chemical brew behind them shapes durability and grip. 4-Methoxy-Aniline-2-Sulfonic Acid plays a supporting role in making certain rubber accelerators. The production chain gets more efficient, and the end results last longer. Companies that rely on predictable performance don’t gamble with unknown suppliers, so stable sources of quality intermediates like this matter. One bad batch, and you might see claims pile up for defective products.
The conversation around these chemicals doesn’t just end with their uses. Environmental groups and regulators demand tight tracking. Sulfonic acids, including this one, can end up in waste streams from production. Strict laws in places like Europe and the U.S. treat safe handling and disposal as non-negotiable. In some cases, wastewater must go through extra steps before leaving a factory. Green chemistry researchers keep looking for safer cousins, but for now, careful stewardship makes sure we keep reaping the benefits of reliable dyes and drugs without turning rivers into chemical dumps.
Anyone who’s watched a factory floor or lab at work sees how small tweaks in chemistry stack up to big changes down the line. Investing time into better filtration systems and training workers on safe handling makes a difference you can measure. Companies that stay ahead of regulations tend to avoid costly shutdowns or recalls. Smart leadership puts money behind both innovation and responsibility. The long view treats intermediates not just as quick fixes, but as building blocks for sustainable growth in everything from clothing to curing disease.
Names in chemistry usually carry clues about a compound’s guts. Look at 4-Methoxy-Aniline-2-Sulfonic Acid. Straight out, the structure combines an aniline core—a benzene ring with an attached amino group—plus a methoxy group at spot four, and a sulfonic acid group parked at position two. Each group brings its own personality, from the reactivity of the amine to the polar punch from the sulfonic acid.
Here’s the chemical formula: C7H9NO4S. Each letter and number stands for a specific type of atom. It’s made up of seven carbons, nine hydrogens, one nitrogen, four oxygens, and a sulfur. The physical make-up delivers a molecule that stands out in water—sulfonic acid groups don’t shy away from a bath. In my time doing lab work, water-loving chemicals tend to be a lot less sticky and a lot more straightforward to clean up.
Tallying up atomic weights from the periodic table, we get the following: carbon brings 12.01 u, hydrogen 1.008 u, nitrogen weighs in at 14.01 u, oxygen 16.00 u, sulfur 32.07 u. Doing the math:
Total: 203.22 g/mol
I’ve watched many a student get flustered trying to memorize weights, but keeping a small periodic table handy never fails.
This molecule pops up most often where color matters—in dyes, pigments, and some specialized chemical processes. That sulfonic acid group does more than just make the molecule soluble; it changes how the compound interacts with other chemicals. Anilines like this one have deep roots in the dye business. I remember one color technology class where we experimented by hooking various groups onto aniline. Small tweaks turned out to produce totally different results in color, brightness, and lasting power.
Chemicals with good water solubility and strong interactions with fabrics make a difference. They latch onto fibers well, boosting staying power. Anilines have seen hard looks for safety, and for a good reason. Certain substitutions raise toxicity, and I’ve seen colleagues in textile labs double-check exposures and push for safer handling or alternatives.
There is always a nudge from users and regulators to use less toxic stuff and cut waste. This nudged some companies to invest in greener versions and closed-loop systems to capture and treat runoff. Using robust waste treatment keeps these strong acids and amines from leaking down the drain. It also helps to keep an eye on new regulations—these shift quickly, especially around compounds used in consumer goods.
Any lab working with compounds like 4-Methoxy-Aniline-2-Sulfonic Acid needs solid safety plans; fume hoods and gloves are not up for debate. Sharing data about exposure and working on cleaner processing technologies can keep staff safer and cut headaches with environmental audits. I’ve seen some groups move to continuous monitoring, which catches leaks early. It’s not just about compliance; people know accidents can carry lifelong consequences.
Researchers dive deep to find replacements or new processes that dial down hazard. Some bright minds even engineer bacteria or enzymes to make similar chemicals in less risky ways. The chemical world never sits still—there’s always another angle to explore, and lessons to share from the bench to the boardroom.
Everyday workplace hazards teach us that even cautious people can run into trouble if they let their guard down. Chemicals like 4-Methoxy-Aniline-2-Sulfonic Acid come with their own baggage. The compound tends to irritate skin, eyes, and respiratory passages. Breathing in dust or letting it touch exposed skin isn't something I’d risk, especially after seeing colleagues deal with the aftermath of careless handling.
Don’t trust that your hands and eyes will recover quickly from accidental exposure. Safety goggles and nitrile gloves make the process less stressful. I put on a lab coat or apron to guard clothing and skin against accidental splashes. In my experience, a properly fitted dust mask or even a respirator brings peace of mind since powders can hang in the air during weighing or transfer.
Goggles and gloves only do so much. If the air gets heavy with chemical dust, everyone in the room shares the risk. Fume hoods have proven their value by pulling contaminants away, especially in shared workspaces. When I worked at a bench without one, I soon realized lingering powder can leave a mild but persistent irritation in the nose and throat.
Labeled, sealed containers make accidents much less likely. Once, a mix-up with unlabeled jars left a team member guessing about cleanup protocol during a minor spill. This compound holds on to moisture and can clump if left unsealed, so keeping it dry, away from direct light, and separate from incompatible substances like strong bases makes sense.
Easy access to eyewash stations and safety showers can save sight and skin. I've seen the difference fast rinsing makes after an accidental splash. People in labs should know where these stations sit, before any work happens. Wash exposed areas with plenty of water, and get help if any bad symptoms don’t clear up.
Not every workday goes smoothly. Small spills respond well to damp towels, picked up carefully to trap powder without raising dust. For bigger disasters, proper containment materials and a spill kit handle the mess. Absorbent pads and neutralizing materials should be ready before you open a chemical bottle. Training all workers helps, because panic never improves cleanup.
Waste from this compound shouldn’t travel down the drain. Local hazardous waste rules protect groundwater, wildlife, and municipal workers. Contacting the right authority or hazardous waste handler prevents careless disposal. At my facility, labeling waste containers with full chemical names avoids confusion when disposal day arrives.
Every step should focus on minimizing exposure, giving coworkers the tools and know-how to deal with problems as they come. Talking honestly about near-misses, updating training, and taking time to maintain gear turns safety rules into habits people follow every day. Investments in proper procedures always cost less than patching up avoidable mistakes, and that holds true no matter which chemical sits on your bench.
From my years working alongside lab techs and chemists, I’ve seen how much can go wrong just by ignoring simple storage steps. Some chemicals only raise eyebrows if they spill or oxidize. 4-Methoxy-Aniline-2-Sulfonic Acid doesn’t explode with drama, but it quietly breaks down or clumps up if left in the wrong spot. Ten years ago, I watched a colleague lose a whole batch of product—not because of sloppy work at the bench, but from humidity slowly creeping into a loosely capped container.
Consistent room temperature helps keep this compound stable. A lot of mistakes start in places without climate control—an old storeroom with heaters blaring in winter, then sweltering in July. The compound doesn’t handle temperature swings, which can encourage unwanted reactions or spoil its purity. Hard-earned lab results can go out the window if a chemical gets ruined before it hits the flask.
The best place is a dedicated, well-shelved storage room—away from direct sunlight and insulated from the building’s wildest temperature changes. Data from MSDS sheets and real-world case studies show even mild heat can speed up decomposition. If storage runs at 20-25°C, you’ve got the sweet spot for storing this type of chemical.
Anyone who’s opened a jar and found a clumped mess knows moisture sneaks in even with short exposures. 4-Methoxy-Aniline-2-Sulfonic Acid, being a sulfonic acid derivative, attracts water from the air. Once it grabs that moisture, you get caking and slow chemical changes. This situation really frustrates anyone trying to weigh out an accurate dose or analyze a clean sample.
From my own time in production facilities, the best results come from using airtight glass or heavy-duty plastic containers. I’ve watched seasoned techs cap bottles right after every use and stash desiccants alongside them. That small habit saves more product than some expensive humidity control setups. Adding a silica gel packet inside storage cabinets won’t hurt, either.
Labs can get cluttered fast, and too many stories start with someone stacking incompatible chemicals side by side. 4-Methoxy-Aniline-2-Sulfonic Acid wants distance from oxidizing agents, strong bases, and metals that might catalyze breakdown. I’ve seen close calls with labels worn off and somebody blindly reaching for the wrong bottle. Clean storage practices—using clear labeling and separate shelves—pay off. Guidelines from OSHA and chemical safety boards back up what common sense already suggests.
Sometimes it only takes a quick inspection to catch problems brewing. I’ve made it a habit to look for signs of moisture, crust, or off smells every month or two. If anything looks wrong, disposal and a fresh order save more money than pushing luck with compromised stock. Whether you’re in a teaching lab or a production line, logging inspections helps everyone stay accountable and reduces surprises.
Sturdy containers and basic climate control offer decent protection. Label everything in plain language, post rules where everyone can see them, and encourage workers to flag anything unusual right away. If funding allows, invest in purpose-built chemical cabinets with humidity control for bigger stashes. Those steps protect both product quality and workplace safety, saving more than they cost over time. Real attention to storage habits turns chemistry from a gamble into a reliable craft.
Sourcing 4-Methoxy-Aniline-2-Sulfonic Acid for lab or industrial uses can sometimes feel simple until you look closer at grades. I've spent time working with chemicals in both research and product development, and purity stuck out as one of those details that shape project outcomes from start to finish.
Purity comes down to more than the label on the drum or jar. The trace stuff left behind during synthesis or transport, how rigorously it’s purified, and even the manufacturer’s process controls — all these factors can shift how the chemical performs later. In my lab days, a subtle difference between a “technical” and a “high purity” grade often meant the gap between clear data and a scrambled mess you’d need to troubleshoot for days.
Not every project calls for the highest specification. A manufacturer making dyes or textile finishes probably works with a technical or industrial grade—enough purity for strong results but not so much that costs spiral up. For pharmaceuticals or analytic work, even a skimpy trace of impurity can throw results or safety off. Academic research often lands in the middle: you want reliability, but budgets won’t always stretch to the ultra-high end.
Data from established suppliers like Merck or Alfa Aesar shows at least three distinct purity designations for this compound. Technical grades tend to hover around 80–90% purity. Lab or analytical grades push the number past 98%. Pharmaceutical or “ultrapure” designations almost always promise over 99% and come with supporting certificates and analytical breakdowns. This is more than marketing — it's assurance when regulations and human safety ride on each batch.
I learned early that small mistakes at the chemical source stage can lead to hundreds of thousands in losses down the road. One project I worked on swapped to a cheaper grade, only to see unexpected foaming ruin a week’s worth of reactors. Only detailed impurity data helped us spot the cause and recalculate our sourcing strategy. Impurities can react, break down, or interfere at critical moments, hitting yields, color stability, safety, and reproducibility hard.
Published recalls and regulatory bulletins back this up. The European Chemicals Agency, for example, tracks cases where wrong grade selection led to product recalls or compliance failures. Proper documentation, supported by independent analyses, has become a must for anyone operating in a regulated field.
Relying on solid documentation stands out as the baseline. Certificates of analysis with batch-level info are non-negotiable for sensitive applications. Buyers should look for supplier transparency: not just a number, but real details about what’s in each drum. On-site audits or third-party inspections help weed out vendors that cut corners.
Building a steady relationship with reputable suppliers pays off. The trust may cost extra upfront, but it dodges the much steeper losses from ruined batches or product recalls later.
Training users, from warehouse staff to lab techs, about why these distinctions matter prevents costly mix-ups. Labels need to stick, records need to match, and everyone needs to understand the “why,” not just the “what.”
Getting the grade wrong might feel easy in theory, but in practice, the stakes are too high. My own experience — and more than a few close calls — keeps me vigilant about purity and supplier track records. The quiet diligence at this early stage sets up everything else to work as expected.
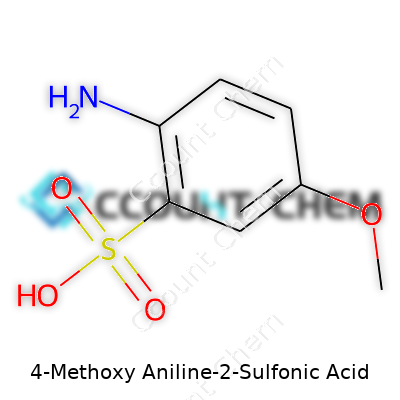
| Names | |
| Preferred IUPAC name | 4-methoxyaniline-2-sulfonic acid |
| Other names |
2-Amino-5-methoxybenzenesulfonic acid 2-Sulfo-4-methoxyaniline 4-Methoxy-2-aminobenzenesulfonic acid 4-Methoxy-o-aminobenzenesulfonic acid Sulfanilic acid, 4-methoxy- |
| Pronunciation | /ˈfɔːˈmɛθɒksi-əˈnɪliːn-tuː-sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 13248-18-7 |
| 3D model (JSmol) | `3D structure; JSmol string: CC1=CC=C(C=C1S(=O)(=O)O)N` |
| Beilstein Reference | 1266999 |
| ChEBI | CHEBI:27736 |
| ChEMBL | CHEMBL463456 |
| ChemSpider | 2021448 |
| DrugBank | DB08365 |
| ECHA InfoCard | 03c8e944-11eb-4e3e-9c10-742ae68e8c50 |
| EC Number | 221-122-7 |
| Gmelin Reference | 73132 |
| KEGG | C14342 |
| MeSH | D014578 |
| PubChem CID | 12391 |
| RTECS number | GV7875000 |
| UNII | 1D97UR5W23 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID9060213 |
| Properties | |
| Chemical formula | C7H9NO4S |
| Molar mass | 287.30 g/mol |
| Appearance | White to light brown solid |
| Odor | Odorless |
| Density | 1.38 g/cm³ |
| Solubility in water | Soluble in water |
| log P | 0.016 |
| Vapor pressure | 0.000071 hPa at 25 °C |
| Acidity (pKa) | -0.7 |
| Basicity (pKb) | 6.04 |
| Magnetic susceptibility (χ) | -57.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Dipole moment | 3.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 232.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -860.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H317 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| Flash point | > 230°C |
| Lethal dose or concentration | LD50 oral rat 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat > 2000 mg/kg |
| NIOSH | NQ8750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | NIOSH does not specify an IDLH value for 4-Methoxy-Aniline-2-Sulfonic Acid. |
| Related compounds | |
| Related compounds |
Aniline-2-Sulfonic Acid 4-Methoxyaniline Aniline Sulfanilic Acid 2-Methoxyaniline 4-Methoxybenzenesulfonic Acid |