The journey of 4-hydroxybenzenesulphonic acid tracks closely alongside the rise of the modern chemical industry. Early organic chemists, working with coal tar derivatives, stumbled upon sulphonation, which paved the way to compounds like this one. In the late 19th century, synthetic dyes and detergents spurred research and patent filings, turning what was once a laboratory curiosity into an important industrial chemical. As companies learned more, they found that adjusting sulfonation conditions shaped the purity and properties, creating room for innovation that fueled new products throughout the twentieth century. Having spent time with chemical patent archives and classic German literature, I've seen how this compound served as both a testing ground and foundation for dye, agrochemical, and pharmaceutical innovations.
4-Hydroxybenzenesulphonic acid, also called p-hydroxybenzenesulphonic acid, delivers more than just a chemical function. Its structure—one hydroxy and one sulfonic acid group fixed to a benzene ring—makes this molecule useful for a range of chemical syntheses. It’s a building block. Whether making azo dyes, intermediates for drugs, or resorcinol derivatives, it's a staple that reappears across decades of chemical manufacturing. From my own experience researching surfactant precursors, this acid’s reliable reactivity simplifies many processes, saving steps that would otherwise involve additional catalysts or tricky handling.
This acid takes the form of colorless to slightly tan crystals, sometimes available as a fine powder. It dissolves well in water, producing a highly acidic solution. With a melting point generally around 100-105°C under proper drying, it resists decomposition until higher temperatures. Its hydroxy group boosts solubility and changes reactivity compared to plain benzenesulphonic acids. The structure allows easier electrophilic substitution, and the acidic sulfonic group enhances its capacity as a leaving group in reactions. During my lab stints, I noticed its tenacity in holding water—keeping a sample dry for analysis takes effort, as the compound picks up atmospheric moisture with ease.
Producers list this compound by purity, typically ranging from 98% upwards for reagent-grade material. Key specifications include melting point, water content, sodium content if supplied as a salt, and iron to ensure minimal contamination. Labels often mention alternate catalog numbers tied to suppliers for quick reference. Packaging uses moisture-proof containers since the compound draws in water. Standard transport labels include UN codes classifying it among low-hazard industrial acids but they note corrosiveness and environmental risks. When reviewing import records and compliance sheets, these details streamline regulatory checks and quality audits.
Manufacturers usually prepare 4-hydroxybenzenesulphonic acid via direct sulfonation of phenol using concentrated sulfuric acid or oleum. The trick lies in temperature control; even a slight shift tilts the balance toward over-sulfonated byproducts or ortho derivatives. Small-batch production can use glass reactors with ice cooling, while larger scales rely on stainless steel or glass-lined equipment with careful monitoring. As someone who’s helped scale-up bench syntheses, I can confirm that mastering this step minimizes waste and tightens cost control—the skill comes in tuning reactant ratios and keeping batch records exhaustive.
The molecule proves wildly versatile. React it with diazonium salts and you get vivid azo dyes, staples of textile and ink industries. Couple it with formaldehyde, and it can yield phenolic resins. Phosphorylation and chlorination open up paths to herbicide intermediates or newer pharmaceutical scaffolds. Its easy compatibility with oxidation, reduction, or coupling reactions means chemists often reach for it when mapping out efficient synthetic routes for newer products. Even during undergraduate projects, I saw firsthand that its dual functionality—the hydroxy and sulfonic acid groups—gives rise to reactivity patterns not easily mirrored by simpler aromatics.
This acid appears under several names: p-hydroxybenzenesulphonic acid, 4-hydroxybenzenesulfonic acid, and parasulfophenol crop up in literature and supplier catalogs. In some regions, trade names might appear, particularly for specialized grades destined for analytics or electronics. The key is always checking CAS numbers and grade statements to avoid mix-ups, because similar-sounding names sometimes point to isomers or related sulfonic acids. Years of combing through chemical catalogues have shown that cross-referencing product codes and synonyms reduces errors and sourcing headaches.
Handling this acid safely calls for goggles, acid-resistant gloves, and fume hoods to dodge splashes and inhalation. Direct contact irritates skin and eyes, and dust breathing can worsen respiratory symptoms over time. Safety data sheets prompt neutralizing spills with soda ash or calcium carbonate and directing wash water into proper acid-neutralization tanks. I’ve seen company training sessions drill these protocols into every lab technician, not just because of regulation, but due to firsthand lessons—minor mishaps prompt real vigilance where strong acids are in play.
This acid forms the backbone of dye and pigment manufacture. It’s a staple in azo dye synthesis, where it helps anchor colorfast pigments onto fabric and paper. Beyond that, its presence emerges in pharmaceutical intermediate production, particularly for sulfa drugs and diagnotic reagents. Some resin and surfactant producers use derivatives to boost wetting or emulsification properties. Its broad acceptance owes a lot to repeatability and compatibility with standard processes. During a consulting project with a dye maker, repeated pilot batches, adjusted only by minor temperature tweaks, all delivered nearly identical yields—rare in complex organic chemistry.
Chemists value this compound as a reference point for testing new sulfonation techniques and green chemistry initiatives. Biocatalytic approaches, alternative solvents, and continuous processing all get bench-tested using it for reliable baseline measures. R&D departments also screen it for making more biodegradable sulfonate surfactants, aiming to shrink their environmental impact. Reviewing conference abstracts and academic papers, I’ve noticed that this acid often anchors comparative studies, mainly because its chemistry is both familiar and slightly challenging, forcing researchers to refine and defend their methods.
Animal studies paint a detailed picture: high doses of 4-hydroxybenzenesulphonic acid cause irritation but don’t show robust signs of carcinogenicity or long-term organ damage at standard exposure levels. Chronic exposure increases risk for dermatitis and respiratory complaints. Environmental release requires tracking, since sulfonated aromatics linger in water and can disrupt aquatic life cycles. Regulatory agencies push for precise monitoring, exposure limits, and documentation of disposal. This vigilance matches what I’ve encountered in compliance audits, where raw effluent logs and medical surveillance records all trace back to these toxicity findings.
Looking ahead, manufacturers and researchers are pressing for cleaner synthesis and improved biodegradability. Demand rises for chemical feedstocks that combine low toxicity, reliable function, and compatibility with circular economy models. Newer enzyme-based sulfonation, process intensification, and hybrid catalysts will probably reshape how this compound gets made and used. Academic collaboration and tighter industry-regulation partnerships drive much of this, as each side seeks to manage risks and protect both workers and the environment. Through direct participation in green chemistry working groups, I’ve seen industry commitment to reducing environmental burden—meeting emissions caps now means rethinking staple compounds like this one from the ground up.
4-Hydroxybenzenesulphonic acid finds its way into more corners of life than most would guess. Some folks might only recognize it as a name on a chemical drum, but without it, a range of products would lose their color, their performance, or even their safety. My own years working in industrial supply opened my eyes to how many chemicals, even ones with science-fiction names, end up connecting to our daily routines.
Walk into a textile factory, and you’ll see why this acid matters. Manufacturers use it as a key intermediate in making dyes—especially azo dyes, which deliver vivid reds, yellows, and oranges. The acid’s structure helps developers attach dye molecules to fabric fibers, turning plain cotton into a shirt that pops with color. Cotton, wool, and even synthetics like nylon often rely on these chemistries. Without chemicals like this, the choices on a clothing rack would look a lot grayer.
Anyone who’s faced a prescription knows the complexity behind every pill. Chemists rely on 4-Hydroxybenzenesulphonic acid during the creation of certain medicines. It takes part in producing drugs for pain relief, treating infections, and several other conditions. The acid doesn’t end up in the final product, but its reactivity speeds up or redirects reactions, saving companies time and resources. Each time a process like this cuts production costs, medicines can reach shelves at better prices.
Take a closer look at household cleaning labels and you’ll spot terms like “surfactants” or “detergents.” This acid acts as a building block for some of these compounds. Thanks to its chemical properties, detergents clean more efficiently, even in hard water. In my own house, laundry and dish soap have to handle minerals that would otherwise leave a step muddy or a glass streaky. The molecular tweaks made possible by 4-Hydroxybenzenesulphonic acid really do make a practical difference.
Municipal water plants rely on more than just big filters. This acid helps create chemicals that bind to contaminants, making it easier to remove them from drinking water. With Indian cities I’ve visited struggling to keep up with demand, the efficiency gained here makes public health less of a guessing game. Safe water, free of heavy metals and organic pollutants, depends on tight chemical processes—4-Hydroxybenzenesulphonic acid supplies part of that.
As much as this compound delivers value, users can’t ignore workplace safety. Like many strong acids, it burns skin and irritates lungs. Suppliers and plant managers bear responsibility for training workers, providing safety equipment, and handling spills correctly. After seeing an accident at a plant early in my career, I never take the risks for granted. Regulators continue to set limits and perform inspections to make sure the cost of chemical progress doesn’t fall on those closest to it.
The world’s appetite for colored clothing, effective cleaners, and cheaper medicine keeps demand for intermediates like 4-Hydroxybenzenesulphonic acid steady. Green chemistry initiatives are pushing companies to refine their processes: recycling reagents, reducing emissions, and shifting to renewable feedstocks. Scientists are even looking for biodegradable alternatives where possible. Making these improvements protects workers and neighbors, and helps keep the products people use safe and affordable.
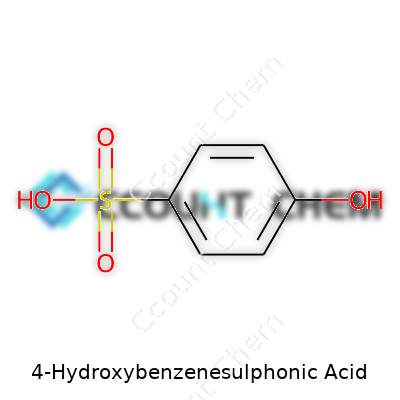
Organic chemistry brings out a fascination I’ve held since my undergraduate days, huddled over a lab bench, hands stained with dyes and fingers crossed that the reactions I’d set up would work as planned. 4-Hydroxybenzenesulphonic acid, with the chemical formula C6H6O4S, stands out not just as another collection of atoms, but as a foundation in aromatic sulfonic acids. Breaking it down: six carbons anchor the benzene ring, each hydrogen and functional group defining the unique characteristics that fuel this compound’s chemistry.
The “4-hydroxy” part tells you a hydroxy group sits on the fourth carbon, relative to the sulfonic acid group. This might sound a bit dry until you’ve held a bottle of the stuff and realized how crucial even small changes on a benzene ring can be. Whether you’re talking pH shifts or solubility, a move from the third to the fourth position changes both outcomes and safety guidelines. This precision gets lost unless you’ve spent hours mapping out resonance structures on scrap paper, but it matters—for labs, for manufacturers, for anyone working with chemicals day to day.
Most people see chemical formulas as cryptic, but formulas like C6H6O4S form the backbone of tasks in dye production, pharmaceuticals, even water treatment. My first chemical job involved testing a batch of azo dyes, and the difference between using 4-hydroxybenzenesulphonic acid and its structural siblings was the difference between a clean synthesis and a mess of byproducts. For companies that crank out thousands of liters of dye or sulfonated intermediates, simple slips can turn profits into headaches.
There’s a wider impact, too. Sulfonic acids like this one dissolve easily in water, which means their handling and disposal have big environmental footprints. If wastewater from a plant isn’t treated to break down organic sulfonates, harmful residues get into rivers and streams. Years back, I watched a municipal meeting unravel when local fish populations dropped and someone traced the cause to wastewaters high in sulfonates. Responsible production starts with knowing exactly what you’re using and how to deal with it safely.
Knowing formulas isn’t arcane trivia for specialists—it’s practical. If you misidentify C6H6O4S, you risk botched syntheses or hazardous waste. Classrooms and businesses both need strong training on how to read and interpret formulas. My colleagues in regulatory agencies stress easy access to chemical databases and real MSDS sheets, since even experts get caught off-guard by mislabeling or outdated specs.
Beyond that, technology plays a role. Automated reaction planning software now builds off precise formulas, helping both small startups and multinational plants avoid costly blunders. Still, none of this replaces the need for hands-on learning and real collaboration. Clear labels and a shared language about chemicals keep things running, which really comes down to education and accountability on the ground—whether in a high school lab or a major plant.
So much of daily industry—and personal safety—hangs on getting the basics right. I’ve seen too many young chemists skip right over the foundation in their rush to handle advanced multi-step syntheses. But the formula for a single compound such as 4-hydroxybenzenesulphonic acid underpins whole careers and whole supply chains. The details matter, and learning to read them correctly sets the stage for safe, productive work in science and industry.
4-Hydroxybenzenesulphonic acid goes by a few different names in labs and factories. It's a white or beige powder with a slightly musty smell, and people use it in dye-making, pharmaceuticals, and as an intermediate in chemical syntheses. Spend enough time around manufacturing, and you’ll see barrels of this acid on the shipping docks. Right away, that tells you it’s pretty common—in small or big operations. Whenever a chemical shows up so often, the question follows: is it safe, or are we playing with fire?
Science offers a guidebook on handling materials like this. Labeling for 4-hydroxybenzenesulphonic acid describes it as an irritant. Skin, eyes, and lungs can react sharply if exposed. I once saw an experienced technician touch his eye after working with a very similar aromatic acid—it led to redness within minutes, even after a rinse. Its powder tends to float in the air during transfer, which makes inhalation a realistic risk in busy labs, especially if there’s no fume hood. Years of safety sheets show the same pattern: keep it off your skin, don’t breathe it in, shield your eyes.
Now, stack this up against true toxins like cyanide. 4-Hydroxybenzenesulphonic acid’s acute toxicity falls in a much milder range. Ingesting it could cause stomach pain, but it’s not deadly at even moderately low levels. Rats used in toxicity studies needed hefty doses before showing systemic distress. That doesn't mean you should snack on it, but spills or dust inhalation are more about short-term irritation than long-term organ damage.
Let’s take it outside the factory. Water and soil can carry all kinds of chemical leftovers. If wastewater full of this acid reaches a river, things get complicated. In my hometown, one pesticide plant dumped wastewater straight into a creek, which led to fish die-offs due to oxygen theft and a stubborn residue on everything. While 4-hydroxybenzenesulphonic acid won’t bioaccumulate like mercury, it can still affect aquatic systems by acidifying water and making fish or invertebrates stressed or sick. Researchers found that even relatively small amounts drop the pH, which triggers the release of metals from the soil. These metals sometimes cause even more trouble for local wildlife.
The risks attached to this chemical respond best to real, hands-on control. Anyone mixing dyes, or manufacturing medicine with this molecule, can lean on gloves, goggles, and proper masks. I worked in a textile plant that swapped open bins for closed auto-feeders. Skin problems dropped by nearly half in a season. Such improvements don’t cost much compared to the expense and headache of dealing with injury reports. Regular exhaust fans, sealed transfer systems, safety showers in reach—all these steps matter more than the dozens of warnings printed on a label.
This story circles back to attention to detail. Workers with proper training, gear, and routines rarely see serious problems. Waste treatment technology can neutralize acids and prevent river contamination—local plants near my city use lime slurries and filtration beds, letting water leave the site cleaner than when it arrived. The right program keeps the environment and people safe while letting industry thrive. Chemical hazards are real, but reasonable respect and simple steps go a long way.
4-Hydroxybenzenesulphonic acid finds its way into plenty of industrial and laboratory settings. Its applications spread across dyes, pharmaceuticals, and chemical research. Its structure, with a sulfonic acid group stuck on a phenolic ring, raises some storage concerns. This compound packs a punch with its strong acidity and a knack for absorbing water from the air. Sweat the details here, because getting lazy about chemical storage—or thinking it’s just another dry bottle—invites trouble.
Anyone who’s dealt with sulfonic acids knows how they can pull water straight from the air. Over time, that humidity leads to clumping, messy spills, or even degradation of the compound. Some folks shrug off a little caking, but wet acid means unreliable lab results, extra cleaning, and higher replacement costs. The chemical draws water almost like a magnet, so give it a dry, cool place to call home. My years working shoulder-to-shoulder with graduate researchers taught me that silica gel packs in chemical cabinets go a long way here. No high-tech fix needed—just an airtight container and consistent monitoring keep moisture at bay.
Hot summers or overheated labs push chemical containers past safe limits. 4-Hydroxybenzenesulphonic acid doesn’t play nice with heat, as higher temps can increase the risk of decomposition and pressure build-up inside sealed containers. This acid prefers stable temperatures, so a dedicated chemical refrigerator away from sunlight works best. Investing in a thermometer for chemical storage rooms might feel old-school, but nobody regrets catching a rising temperature before something goes wrong. Back in my own small workshop, I caught a costly spill once from a forgotten bottle left near a sunny window—never made that mistake again.
Rushing to pour a new delivery of acid into a random jar creates confusion and raises the risk of cross-contamination. Keeping 4-Hydroxybenzenesulphonic acid in its original, labeled container helps everyone steer clear of mix-ups. Proper labeling with hazard symbols lets even new staff members know what they’re handling. I’ve watched new techs frown at unlabeled powders, hesitating to work until a supervisor arrived. Clear, honest labeling and records turn confusion into safety.
This acid doesn’t release dangerous fumes under normal conditions, but leaks or spills can irritate skin and mucous membranes. Adequate ventilation, with storage away from busy hallways or direct draft from vents, keeps the workspace healthy. Every facility benefits from spill kits ready and easy to grab—absorbent pads, gloves, and goggles matter more than most realize. Thinking long-term, it pays to review spill protocols with everyone, from new hires to managers. I’ve been in workshops where quick action saved both health and equipment from lasting damage.
Leaving hazardous chemicals unlocked increases the risks of theft, misuse, and accidents. Lockable cabinets mean no surprises after hours, and regular inventory checks stop expired or degraded products from lingering undetected. Even the best labs slip up if no one’s assigned to check expiration dates or look for leaks. Transparency about chemical storage—shared logs, checklists, and a culture of open communication—saves time and reduces errors. In my experience, a quick morning audit prevents the headaches a missed issue causes down the line.
Safe storage of 4-Hydroxybenzenesulphonic acid isn’t just a best practice. It’s an everyday responsibility. Dry, cool conditions, airtight containers, precise labeling, and trained staff keep this compound working as it should without risking health or research results. Setting up clear routines and treating each bottle with the respect it deserves reflects professional pride and safety-first habits.
4-Hydroxybenzenesulphonic Acid stands out for its practical usefulness and shows some pretty interesting physical features. Let’s cut to the chase—this compound shows up as a solid. Most chemical suppliers sell it as a white to light beige crystalline powder. I’ve seen this on the shelves of university labs, and the pale color always makes it easy to spot. As someone who’s spent time in research settings, the acid’s manageable form means fewer headaches for weighing, measuring, or transporting.
No chemical conversation gets far without talking about water solubility. Drop a spoonful of this compound in water and it dissolves quickly. That’s a big deal for folks working in dyes or pharmaceuticals, where you don’t want granules sticking around. Its sulfonic acid group helps in binding with water molecules, so you’re left with a clear, even solution instead of murky sludge. There’s not much smell either—nothing strong or choking in the air—which sets it apart from many other chemicals that can empty a room.
Where heat is concerned, 4-Hydroxybenzenesulphonic Acid has a melting point reported between 110°C and 112°C. Tossing it into the furnace won’t reduce it right away to a mess, but push past that and you see changes. The fairly modest melting point compared to similar acids is something to keep in mind, whether you’re making pigments or running a high-temperature reaction. I’ve seen lab techs keep a closer eye on temperature dials with this one on the bench.
Feel the powder between your fingers and you’ll sense a fine, free-flowing nature. In daylight, the crystalline structure reflects a soft sheen, nothing glossy or sticky. That lack of stickiness keeps it from lumping together, which is no small feat if you’re moving it in bulk. Aging warehouses and drafty science departments both benefit from this—it stores well under dry conditions and won’t clump up like sugar on a damp day.
This acid gets its bite from the sulfonic group attached to the benzene ring. On the pH scale, it lands deep in the acidic range, somewhere near 1. Such a strong acid needs proper handling. I’ve seen students pinch their finger gloves a bit tighter when prepping a beaker. Direct contact is a no-go for exposed skin. Standard lab safety makes sense here: gloves, eye protection, and a fume hood if you’re adding heat.
The practical side can't get ignored. 4-Hydroxybenzenesulphonic Acid doesn’t need refrigeration and keeps its quality for a long while as long as it stays dry. That reliability attracted the dye industry a century ago, and not much has shifted since. In smaller setups or small-scale projects, that kind of shelf stability reduces waste and cost.
Strong physical properties make 4-Hydroxybenzenesulphonic Acid a workhorse in fields ranging from textile to pharmaceuticals. Water-friendly, stable in storage, and strong as an acid—these aren’t just bullet points on a data sheet. They shape how safely and effectively scientists get work done. Taking a few simple steps—dry storage, personal protection, good ventilation—keeps labs safe and keeps experiments rolling, year after year.
| Names | |
| Preferred IUPAC name | 4-hydroxybenzenesulfonic acid |
| Other names |
Benzenesulfonic acid, 4-hydroxy- p-Hydroxybenzenesulfonic acid Sulfanilic acid 4-Hydroxybenzenesulfonic acid p-Hydroxybenzenesulphonic acid |
| Pronunciation | /ˈhʌɪdrɒksiˌbɛnˈzoʊnˌsʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 98-67-9 |
| Beilstein Reference | 802938 |
| ChEBI | CHEBI:27859 |
| ChEMBL | CHEMBL58082 |
| ChemSpider | 15780 |
| DrugBank | DB03765 |
| ECHA InfoCard | 03c8d8fd-54d8-44f3-9e06-4aaf60fff8b2 |
| EC Number | 3.1.6.1 |
| Gmelin Reference | 126008 |
| KEGG | C02521 |
| MeSH | D016207 |
| PubChem CID | 7410 |
| RTECS number | DA4375000 |
| UNII | 380P07092K |
| UN number | UN2585 |
| Properties | |
| Chemical formula | C6H6O4S |
| Molar mass | 174.18 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.34 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.0 |
| Vapor pressure | 0.0000127 mmHg at 25°C |
| Acidity (pKa) | 1.0 |
| Basicity (pKb) | 6.8 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.6180 |
| Viscosity | 1.04 mPa.s (20 °C) |
| Dipole moment | 3.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 197.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -642.7 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -790 kJ mol-1 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H318 |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Flash point | 138°C |
| Autoignition temperature | Autoignition temperature: 605°C |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid p-Cresol 4-Aminobenzenesulfonic acid Phenol Sulfanilic acid 2-Hydroxybenzenesulfonic acid |