Chemistry has a habit of building on old foundations. 4-Hydroxybenzene sulfonic acid traces roots to the drive for synthetic dyes in the late 19th century, back when chemists began to see the untapped potential in modifying benzene rings. Around the 1880s, German researchers tinkered with aromatic sulfonation, producing a variety of sulfonic acids. These grew up alongside the textile industry, where demand for more vibrant and colorfast dyes pressed chemists to explore new reagents. The presence of both hydroxyl and sulfonic groups on a benzene ring turned out to be a sweet spot for solubility and reactivity, making this molecule a building block in the evolving world of synthetic organic chemistry.
4-Hydroxybenzene sulfonic acid falls into the class of phenolic sulfonic acids. It’s known for high water solubility, a direct result of its sulfonic acid group, and it tends to show up as colorless to slightly yellowish crystals or powder, depending on purity. This compound is valued in both research and industry, and its versatility has kept it in demand, especially for those working in dyes, pharmaceuticals, and specialty chemicals.
This solid typically melts around 103–105 °C, forming fairly stable crystals under ambient conditions. Its solubility in water stands out—most sulfonic acid derivatives dissolve readily and that’s certainly true here, with completely clear solutions at standard concentrations. A strong acid nature shows itself in quick, complete dissociation in water, and the free phenol group offers potential for further transformation. It's stable to ordinary handling, but on heating, decomposition brings some classic sulfur dioxide smells, warning that high temperatures push things too far.
Labs and suppliers catalog this compound by multiple product codes and usually denote content above 98% for research-grade quality. Labels carry hazard statements about skin and respiratory irritation. Many register this substance under the globally harmonized system (GHS), using identifiers like UN numbers and specific pictograms. Researchers and workers read these specs to know what to expect during handling, shipping, or disposal.
Industrial synthesis mostly follows the sulfonation of hydroquinone. Technicians mix hydroquinone with concentrated sulfuric acid, controlling temperature to encourage the para-substitution rather than ortho. I have seen technicians in university labs measure temperature closely in an ice bath just to slow down the reaction—exothermic surges can char the whole mix if not watched. Many modern plants automate this step, but anyone making it by hand recognizes the importance of slow acid addition and efficient cooling.
Once in hand, chemists use 4-hydroxybenzene sulfonic acid as a launching pad for more complex products. Typical reactions involve esterification, where the sulfonic group forms simple or mixed sulfonate esters, or alkylation to build up the phenol ring. This compound reacts directly with strong bases to form water-soluble salts—sodium salt being the most popular for batch operations. I’ve seen researchers introduce diazotization steps for dye production, or attach different alkyl chains for specialty surfactants; the versatility supports a range of downstream chemistry.
Names pile up across catalogs: 4-hydroxybenzenesulfonic acid, p-hydroxybenzene sulfonic acid, para-hydroxybenzenesulfonic acid, and even p-phenolsulfonic acid. Sometimes suppliers shorten to HBS acid. Chemical Abstracts Service tracks it by registry number for accuracy. Each name traces back to the same basic structure—hydroquinone sulfonated at the para position—so ordering from different suppliers mostly means cross-checking purity or grade.
Workplace safety starts with respect for strong acids. 4-Hydroxybenzene sulfonic acid irritates skin, eyes, and lungs on contact, so gloves, goggles, and fume hoods make routine companions. Material safety data sheets rate it as hazardous for inhalation and direct contact but manageable with modern PPE and exhaust ventilation. Proper storage keeps the powder dry and away from incompatible bases and oxidizers; accidental mixing leads to heat and gas release. Regular training teaches staff to handle spills and dispose of waste through regulated facilities—not down the sink, no matter how dilute.
Most end-users see this compound as a key intermediate. In dye chemistry, it helps create vivid azo and anthraquinone colors, boosting solubility and fiber affinity. Paper and leather processors use it to prep surfaces or introduce chemical resistance. In the drug industry, it acts both as a raw material for sulfa-related drugs and sometimes as a catalyst or protective agent during multi-step syntheses. Water treatment professionals look for the sodium salt as a dispersing agent or as an additive to control scale and fouling. Back in the lab, I've often seen it feature in polymer chemistry, where sulfonated systems demand reliable sources of aromatic acid groups.
The pursuit of greener production drives most new research, given the energy and waste footprint of traditional sulfonations. Some teams push enzymatic or milder catalytic methods, hoping for the same yields without harsh reagents. Analytical chemists focus on trace analysis in complex mixtures, refining HPLC and mass spectrometry protocols for environmental monitoring. On the application side, researchers investigate modified derivatives for better antimicrobial action or improved electronics performance, especially in polymer electrolyte membranes. At conferences, folks debate whether the extra cost of green synthesis pays off in the volume market, but the push toward cleaner chemistry shows up everywhere.
Toxicology reports show that 4-hydroxybenzene sulfonic acid poses moderate risks with acute exposure, bringing irritation but not outright lethality at low concentrations. Chronic exposures raise some concerns over persistent respiratory issues and potential skin sensitization. Animal studies find low oral toxicity, but repeated contact or inhalation prompts stricter limits in occupational settings. Environmental researchers note its ready biodegradability compared to some aromatic acids, yet caution against unchecked waste streams. Safety limits follow recommendations from organizations like the ACGIH and NIOSH; personal experience emphasizes following gloves-and-respirator routines in any open handling.
With the chemical industry seeking more sustainable intermediates and less environmental impact, demand for alternatives or improvements involves both process technology and product design. Many research groups now test catalytic sulfonations that require less sulfuric acid, sometimes switching to continuous flow methods that yield less waste. Opens up prospects for bio-based aromatic feedstocks, replacing petrochemistry for the benzene core. In applications, new polymers and battery technologies push for functionalized aromatic acids, and this compound remains a template for sulfonation chemistry. Policy changes and consumer preferences for greener chemistry may shift supply chains, giving space for startups and established firms alike to experiment with both greener manufacturing and circular recycling models. I see potential in collaborative R&D where waste valorization meets new material science—the future of this molecule ties tightly to the larger move toward responsible, efficient chemical processes.
4-Hydroxybenzene Sulfonic Acid sounds technical, but you’ll find its impact in everyday products and crucial industrial processes. Working in chemical manufacturing for years, I’ve watched this compound move off the supply shelves into practical uses, each one touching daily life and vast sectors.
Many people don’t realize the science layered into the colors they see on fabric, leather goods, and paper. The dye industry makes regular use of 4-Hydroxybenzene Sulfonic Acid for its ability to step up the performance of azo dyes. This acid helps anchor color molecules to fabric, so clothing stays vibrant wear after wear—no more faded reds after two trips through the wash. Studies highlight how sulfonic groups on aromatic rings boost color strength and enhance water solubility for dyestuffs; this translates directly into lasting, intense color seen on retail shelves and uniforms alike.
Out on the pharmaceutical floor, innovation doesn’t slow down. Chemists use compounds like 4-Hydroxybenzene Sulfonic Acid to synthesize active pharmaceutical ingredients and intermediates. It supports sulfonation reactions that produce molecules with real therapeutic potential. For example, some antibiotics and antipyretics reach their final mold with help from this compound. Its structure allows flexibility in drug formation, so researchers deliver medicines with fewer side reactions. The same approach drives forward improved painkillers and treatments for chronic diseases. Rigorous quality checks confirm that pharmaceutical-grade batches match strict health and safety standards.
Walk into any office or classroom and you’ll see paper everywhere. Paper manufacturers value 4-Hydroxybenzene Sulfonic Acid because it acts as a dispersant and helps bind optical brighteners. In papermaking, fiber dispersion matters. This chemical makes it easy for factories to spread additives evenly through wood pulp. High-performance brighteners latch onto sulfonic groups, giving paper that clean, white look so prized in printing and publishing. Data from paper trade organizations show that steady use yields fewer rejects and saves energy per ton compared to older formulas.
Beyond paper and textiles, specialty resins and additives get their distinctive properties with the help of this compound. Resin producers rely on sulfonation steps driven by 4-Hydroxybenzene Sulfonic Acid to build strong, heat-resistant structures, especially where electrical insulation is concerned. Additives built on this molecule improve performance in rubber and specialty plastics, creating products able to stand up to stress, UV light, or corrosive environments. In the lab and on the factory line, reliable access to pure chemicals means fewer breakdowns in electronics and safer finishes in construction.
As demand rises in high-tech sectors, stewardship around this chemical grows more important. Regulatory bodies keep standards tight. Suppliers invest in cleaner production and safe handling, recognizing the importance of sustainability. Professionals, myself included, emphasize regular training and proper containment, since accidents with sulfonic acids can pose risks to workers and communities. Responsible use allows industries to keep benefiting from its versatility while protecting health and the environment.
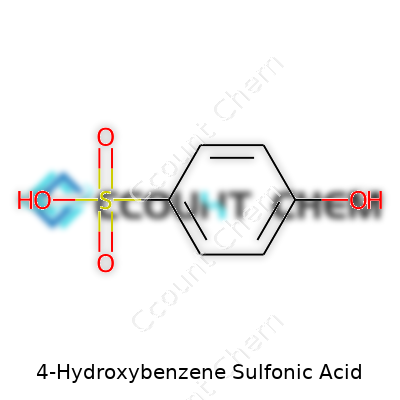
Every time research labs try to predict how a molecule behaves, they focus closely on its chemical formula. 4-Hydroxybenzene sulfonic acid doesn’t appear out of thin air—it comes from basic building blocks that determine its power in industry and research. The chemical formula stands as C6H6O4S. For those who remember some high school chemistry, this formula unfolds to a simple but telling message: six carbons form the aromatic ring, six hydrogens bring both hydrogen-bonding and reactivity, four oxygens tie into both the hydroxyl and sulfonic acid groups, and sulfur forms the backbone of the sulfonic portion.
The molecular weight of 4-hydroxybenzene sulfonic acid lands at 174.18 g/mol. This number doesn’t just satisfy curiosity; it matches with real needs in the field. In my own bench experience, even the tiniest miscalculation in molecular weight throws experimental results out the window. Imagine a chemist weighing out this compound for a calibration standard. If they guess wrong, their whole chain of measurements falls apart. Pharmaceutical companies lean hard on this kind of accuracy too. When a molecule shows up in drugs or as a starting point for other chemicals, knowing its exact mass makes the difference between a batch that passes inspection and one destined for disposal.
4-Hydroxybenzene sulfonic acid isn’t just a name—it’s a promise of certain behavior in the lab or factory. The hydroxyl group (–OH) attached to the benzene ring brings some key advantages. It boosts solubility in water, making this compound easier to use in aqueous reactions. The sulfonic acid group (–SO3H) is infamous for its acidic punch, providing a low pKa. Dye makers and water treatment engineers know this trait well, as it leads to strong ion exchange properties. Sulfonic acids, including this one, help detergents cut through oily grime, and they stabilize dye solutions.
I’ve watched manufacturers use this compound to improve the brightness of certain dyes, and I’ve seen how the hydroxyl group helps make binding to metals more predictable. In waste treatment, it helps break down tricky pollutants; the strong acid group plays a key role in that breakdown process.
4-hydroxybenzene sulfonic acid works well, no question, but handling it safely calls for good lab habits. Its corrosiveness means gloves and goggles aren’t optional. Spills can damage skin and sensitive equipment. Access to up-to-date Safety Data Sheets (SDS) helps prevent accidents. Training isn’t just a box to check—it’s a real world difference between routine production and workplace injury.
Waste disposal brings another set of challenges. This compound, like many sulfonated aromatics, calls for careful neutralization. Improper disposal leads to water contamination down the line. Companies step up by treating waste before release and looking for ways to recycle process water. In my own time working with sulfonated organics, I’ve found that simple steps—double-checking waste containers, testing neutralization with pH paper—spare headaches and regulatory trouble.
There’s a bigger question at play: how can chemists and companies minimize risk from these strong acid compounds? Green chemistry trends push for chemicals with similar benefits but lower toxicity and easier breakdown in the environment. Investment in closed-loop systems keeps handling safe and reduces waste. Working with accurate data, including the correct formula and molecular weight, keeps these transitions cost-effective and trustworthy.
4-hydroxybenzene sulfonic acid supports plenty of important processes. Understanding its structure, weight, and practical hazards turns it from just another name in a handbook to a real-world building block that deserves respect at each step.
Most folks never run into 4-Hydroxybenzene Sulfonic Acid at the grocery store, but chemists and factory crews know it well. Factories use it for dyes, paints, and sometimes in fancy chemistry projects. Getting technical, this compound comes from benzene and brings both a sulfonic acid group and a hydroxy group onto its structure. Its name doesn’t exactly roll off the tongue, yet people in industry circles keep it handy for its strong acidic properties.
I remember walking into my first college synthesis lab. Our instructor made sure gloves and goggles stayed on – no exceptions. 4-Hydroxybenzene Sulfonic Acid reminds me why. Like many organic acids, its powder and solutions try to sting the skin. If you let your guard down and get some on your fingers, you’ll feel it. Spills can spark irritation or rashes, and breathing in the dust feels even worse for your nose and lungs.
Nobody plans to taste a lab chemical, but accidents happen. Swallowing even a little can bring on nausea, cramps, and a burning mouth. From what I’ve seen and learned, eyes need just as much protection—splashes hurt and sometimes cause lasting damage if you delay rinsing. I once watched a student hurry to the eyewash after a similar acid landed in his eye. He walked away okay, but shook up.
Factories store 4-Hydroxybenzene Sulfonic Acid in drums. Leaks, even tiny ones, mean acid fumes get into the workroom. Over time, constant breathing of such fumes doesn’t do lungs any favors. Bad habits—like skipping a mask—add up. My family worked in chemical plants over the years, and hearing stories of coughing fits and trips to the clinic came as no surprise. Long-term exposure has more risks for folks with asthma or weaker immune systems.
People often forget that what falls down the drain rarely vanishes. 4-Hydroxybenzene Sulfonic Acid sticks around in water and soils, and its sulfonic group doesn’t break down easily. Streams and rivers pick up industrial runoff, which means fish and frogs end up in contact with things they never adapted to handle. Even low levels mess with aquatic life, so strict waste controls matter. My time volunteering with a local river cleanup opened my eyes to how little chemical waste it takes to change a stretch of water.
Practical safety means routine checks: goggles, gloves, masks, and solid training. Factories using this acid set up containment trays, fume hoods, and clear labels so no one grabs the wrong bottle in a rush. Sending factory teams through yearly chemical handling classes keeps rookie mistakes low. For cleanup, neutralizing spills right away stops further spread. Outside the plants, lawmakers hold people accountable by setting legal discharge limits into water and air.
Chemistry keeps life humming along: bright dyes, shiny papers, clean circuits. Yet, no shortcut replaces smart, strict safety. 4-Hydroxybenzene Sulfonic Acid teaches us to earn trust by proving that safety wins every shift, every time.
Anyone who's spent time in the lab with aromatic sulfonic acids learns quickly they behave quite differently from simple organic compounds. Take 4-hydroxybenzene sulfonic acid, for example. It looks unassuming as a crystalline powder, but plenty of researchers and industry folks know it for one main reason: It dissolves almost instantly in water. Watching it disappear with a quick stir makes a strong first impression. Turns out, that strong sulfonic acid group and the extra hydroxyl on the ring pack a double punch, making it incredibly hydrophilic.
A big part of the story comes from the structure. 4-Hydroxybenzene sulfonic acid, a derivative of phenol, carries both an -OH group and a -SO3H group. Both can form hydrogen bonds with water, multiplying their ability to attract water molecules. Sulfonic acids, in general, rank among the most water-soluble organic acids people work with. Literature numbers back this up, regularly citing values over 1000 g/L at room temperature. To anyone running color-formulation or dye chemistry, such solubility means direct, smooth mixing and fewer headaches around precipitation.
But water isn’t everything. Folks sometimes want this compound in organic solvents — maybe to adjust a formulation or work around a water-sensitivity issue in synthesis. Here’s where things change direction. Sulfonic acids typically resist dissolution in solvents like toluene, chloroform, benzene, or even diethyl ether. They just don’t get along with nonpolar environments. Throw 4-hydroxybenzene sulfonic acid into ethanol and you’ll see a very modest amount dissolve; step up to methanol, and limited solubility kicks in thanks to more hydrogen bonding. Once, during a project blending phenolic sulfonates into a methanolic solution, I saw the mixture cloud up fast — it just wouldn’t keep everything dissolved. In acetone or isopropanol, almost nothing goes into solution.
The high water solubility of 4-hydroxybenzene sulfonic acid changes how companies treat these materials during manufacturing. For dye intermediates, water-based workups and formulations reduce the risk posed by flammable solvents, keep costs down, and limit environmental impact. The trade-off? It becomes much harder to extract these acids from aqueous mixtures with organic solvents. Purification gets more complex without good partitioning, which slows down both research and production.
It is essential to double-check the purity of commercial samples, especially after long storage in humid conditions, because these compounds pull water from the air easily. Hydrating or clumping can change the measured weight, skewing batch records and reactions. For anyone writing analytical procedures or regulatory filings, tracking and reporting water content in these acidic powders helps avoid downstream quality issues.
Simple changes make a difference. When water removal becomes necessary, chemists lean on vacuum drying or desiccators. For application in less polar environments, using salt forms (like sodium or potassium 4-hydroxybenzene sulfonate) can sometimes bridge the gap, since their interaction with water acts a bit differently and can alter crystallization. Once, in an R&D lab job, we found that introducing a slight pH change with a buffer not only improved recovery of our active ingredient but also solved stubborn solubility problems during scale-up.
Future work will focus on finding co-solvents or surfactants that let 4-hydroxybenzene sulfonic acid move into less polar media — or even tailoring its molecular structure to fit new applications. How we handle water-loving compounds has a real impact on process safety and efficiency; taking time to understand the science behind solubility leads to better products and fewer manufacturing surprises.
4-Hydroxybenzene sulfonic acid isn’t a name most people use in daily life. This chemical plays a role in manufacturing dyes, pharmaceuticals, and other specialty chemicals. With its uses, safety should remain a priority—both for the people who handle it and anyone who works near a storage area.
Chemicals bring many benefits, but only if handled with respect. Keeping this acid in a cool, dry spot keeps things stable. Heat disrupts its chemical structure, raising risks of unwanted reactions, so the temperature should stay below common room temperature if possible. I’ve seen what can happen when facilities overlook simple steps: a little bit of moisture sneaks in, and before long, the product clumps up or even leaks through weak packaging. Using airtight containers like glass jars or corrosion-resistant plastic drums gives much better results than relying on flimsy packaging. Labeling helps everyone avoid confusion, especially since warehouses tend to get crowded.
Direct contact irritates the skin and eyes and creates problems you’d rather avoid. Sometimes people skip gloves “just this once,” then spend the rest of the day washing their hands over and over because of a persistent itch. Nitrile gloves and splash goggles cut down on these worries. Getting good airflow in the storage area stops fumes from building up. If someone spills some powder or solution, there’s little time to waste; absorbent pads and neutralizing agents make clean-up quicker. I’ve worked in labs where early preparation made a huge difference. You never feel completely ready for spills until you’ve handled one, but access to spill kits and a clear plan helps even rookies step up under stress.
Nobody makes smart decisions if they don’t know what’s at stake. Regular training refreshes simple points: don’t store acids near bases, don’t mix chemicals without thinking, don’t open containers without eye protection. Safety data sheets explain which side effects might show up. Knowing the right first aid steps counts just as much as using the right gloves or shields. I remember a coworker who once tried to “shake out” a container he thought was empty, and a fine mist got in his eye. He ignored the warning signs at first, thinking it was just dust, and landed in the nurse’s office. That day, the whole crew started treating those sheets with more attention.
No one likes dealing with chemical waste, but improper disposal leads to headaches for everyone nearby. Neutralize wastes according to local regulations and always keep them apart from regular garbage. If things go wrong—fire, leaks, accidental ingestion—emergencies demand set procedures. Quick-access eyewash stations, emergency showers, and fire extinguishers make a big difference. Drills never seem important until real problems arise, but they shape how people act when every second matters. Using backup plans and sticking to safety basics works better than hoping nothing ever happens.
Chemicals like 4-hydroxybenzene sulfonic acid don’t reward shortcuts. Care pays off over time, reducing accidents and securing both personal health and workplace safety. The right gear, clear plans, and open communication make the difference between routine work and risky business. Learning from mistakes—yours or someone else’s—remains the best way I know to protect everyone who steps inside a chemical storage room.
| Names | |
| Preferred IUPAC name | 4-hydroxybenzenesulfonic acid |
| Other names |
Benzenesulfonic acid, 4-hydroxy- p-Hydroxybenzenesulfonic acid Sulfanilic acid 4-Hydroxybenzenesulfonic acid p-Hydroxyphenylsulfonic acid |
| Pronunciation | /ˈfɔːr haɪˈdrɒksɪˌbɛnˈziːn sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 98-67-9 |
| Beilstein Reference | 1720183 |
| ChEBI | CHEBI:28456 |
| ChEMBL | CHEMBL490323 |
| ChemSpider | 10550 |
| DrugBank | DB03824 |
| ECHA InfoCard | 03-2119457563-43-0000 |
| EC Number | 232-189-2 |
| Gmelin Reference | 9079 |
| KEGG | C02323 |
| MeSH | D017407 |
| PubChem CID | 979 |
| RTECS number | SL9625000 |
| UNII | 0T6FQ3GB14 |
| UN number | UN2585 |
| CompTox Dashboard (EPA) | DTXSID8020793 |
| Properties | |
| Chemical formula | C6H6O4S |
| Molar mass | 174.17 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.574 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.1 |
| Vapor pressure | 3.1 x 10^-7 mmHg (25°C) |
| Acidity (pKa) | 1.0 |
| Basicity (pKb) | -6.3 |
| Magnetic susceptibility (χ) | -49.0e-6 cm³/mol |
| Refractive index (nD) | 1.585 |
| Dipole moment | 4.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 191.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -604.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2044 kJ/mol |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H318: Causes serious eye damage. |
| Precautionary statements | P280-P264-P305+P351+P338-P301+P312-P304+P340 |
| NFPA 704 (fire diamond) | 1-0-0-A |
| Lethal dose or concentration | LD50 oral rat 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | NA8180000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended): 5 mg/m³ |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid p-Cresol Phenol Sulfanilic acid 2-Hydroxybenzenesulfonic acid |