Looking through the lens of chemical progress, 4-Hydroxy-1-Butanesulfonic Acid turned more than a few heads once its unique properties came to light. Sulfonic acids, in general, have long played a part in everything from dyes to drug synthesis, but the attachment of a hydroxy group at the four position on a butanesulfonic backbone offered something new. Back in the late twentieth century, innovative chemists recognized its potential not just as an intermediate but as a building block for more specialized processes, especially in the fast-evolving pharmaceutical and electrochemical industries. Over time, research teams across Europe, North America, and East Asia refined the synthesis routes, moving from batch reactions to more controlled, higher-yield continuous processes to answer industrial demands.
4-Hydroxy-1-Butanesulfonic Acid catches attention because of its useful combination of a sulfonic acid group and a hydroxyl group on a straight-chain four-carbon base. These features let it act as both a hydrophilic modifier and a functional handle in multi-step synthesis. Demand comes from fields where solubility and ionic strength tweaks make or break a process, such as buffer systems, chromatography, custom surfactant formulations, and tailored electroplating baths. It’s not only sold as a reagent for research-level applications but also finds steady purchase for pilot and commercial-scale production batches in select markets.
This compound typically appears as a white or off-white crystalline solid but, depending on manufacturing details or trace impurities, could shift to a viscous liquid. Its molecular weight sits at 154.18 g/mol. It shows good solubility in water, reflecting the influence both of the hydroxyl and sulfonic acid functionalities. Its melting point often falls in the range of 105–110°C. The acid group brings strong acidic character, measured by a low pKa, so in solution it donates protons readily and can adjust pH in buffered systems with accuracy. When kept cool and dry, it maintains its stability; exposure to high humidity for too long might lead to gradual caking or decomposition.
Quality producers list clear technical specs: assay levels consistently over 98% (with high-purity grades clearing 99%), sulfates and heavy metal content kept below 0.01%, moisture content below 1%. Labels must give the IUPAC name, CAS Number 13360-57-1, batch number, net weight, and manufacturer info. Current safety regulations demand extra line items for chemical hazard pictograms, risk and safety phrases, and appropriate hazard codes. Batch-specific data sheets tie together traceability, purity, and recommended storage conditions. These practices grew tighter with the reach of international safety certifications such as ISO 9001 and changes to REACH or GHS requirements over the past two decades.
A favorite preparation method uses 1,4-butanediol through sulfonation, often with sodium (meta)bisulfite under controlled heat. This approach manages solid-liquid phase challenges at scale and keeps byproducts down. More refined labs take extra steps to minimize trace metal contamination using glass-lined reactors, and several patents outline subtle tweaks in catalyst or process control. Once the reaction completes, acidification and careful washing pull out excess salts, followed by recrystallization or drying steps if the industry needs a higher specification. Sharing shop talk with anyone who’s run these batches, most would agree that safe handling of strong acids and careful venting are critical, as are post-synthesis purifications to reach tight specs for specialty uses.
The dual functional groups on 4-Hydroxy-1-Butanesulfonic Acid open the door for plenty of downstream chemistry. The hydroxyl site reacts via esterification or etherification, introducing a wide range of substituents for application in pharmaceutical intermediates or tailored surfactant systems. The sulfonic acid group partners up in salt formation, leading to sodium, potassium, or ammonium salts with tuned solubility profiles. Some processes protect one group while modulating the other, relying on selective protection and deprotection strategies common in organic synthesis. Over years in the lab, chemists see just how much mileage comes from being able to tweak hydrophilicity or reactivity with standard modifications, especially when scaling up for complex molecule production.
Ask around chemical suppliers or scan technical data sheets, and a few aliases pop up regularly: 4-Hydroxybutane-1-sulfonic acid, Gamma-hydroxy-n-butanesulfonic acid, or more systematically, 4-Hydroxybutanesulfonic acid. Sometimes the sodium salt pops up as Sodium 4-hydroxybutane-1-sulfonate. These differences can trip up procurement or regulatory compliance, so any decent operation sticks close to CAS registry numbers and checks translations carefully across safety forms. Branding in the reagent industry varies—some trade-focused distributors assign proprietary product codes or minor spelling tweaks to stand out in catalogues.
The acid brings the usual warnings for corrosive substances. Direct contact stings, so users need gloves, goggles, and fume hood precautions. Inhalation risk runs low at standard temperatures, but dust or mist hazards still require a good mask and solid ventilation. Many storage rooms carry dual controls for temperature and moisture because too much humidity accelerates clumping or breakdown, and any split containers spell trouble fast if they come near incompatible alkalis or oxidizers. Disposal protocols call for dilution, neutralization, then authorized waste collection—cutting shortcuts risks not just regulatory trouble but downstream water contamination. Historically, a few production plants found out the hard way that improper waste handling invited both legal action and public backlash.
Fields using 4-Hydroxy-1-Butanesulfonic Acid benefit from its ionic strength and hydroxy functionality. Electrochemical plating outfits rely on it as a bath additive to manage coating structure and brightness. Pharmaceutical labs test it as a counterion or buffer in both research and pilot scale synthesis. Specialized chromatographers appreciate how it boosts resolution when separating charged biomolecules or basic peptides. Its strong acidic nature serves in pH control for certain fermentative or enzymatic processes. Environmental specialists explore its use in tailored formulations to capture heavy metals or restrict unwanted byproducts. The reach grows as more scientists test it under new conditions and expand its workload.
Recent years brought an uptick in grant support targeting sulfonic acid derivatives. R&D labs push advances in green chemistry, trying to swap toxic or high-impact reagents with safer alternatives. Teams in Japan and Germany led the way with continuous flow syntheses that improved efficiency and lowered waste. Drug discovery programs see value in using the acid or its derivatives to control solubility and stability profiles of target molecules. Electrochemical research circles back again and again to subtle modifications in the hydroxybutane skeleton, hoping to outperform legacy additives under next-generation power storage conditions. Looking through patents and papers from 2015 on, there’s a clear competition to shorten synthesis steps, lower contamination, and integrate real-time analytical feedback in production.
Biochemical safety tests on 4-Hydroxy-1-Butanesulfonic Acid track effects on mammalian cells and aquatic environments. So far, acute toxicity stands at moderate levels, with recommended exposure limits and spill control drawn from in-vivo rodent assays and modeled ecosystem impacts. In the lab, chronic exposure brought on minor dermal or respiratory symptoms, so handling instructions stick with tried-and-true best practices: gloves, goggles, and good ventilation. Environmental fate studies reveal it breaks down eventually under biological conditions, but residual sulfonates can linger if dumped in large amounts, affecting downstream aquatic species. Regulatory agencies keep the thresholds low, and major industry players keep up-to-date with their material safety data sheets as new toxicological findings roll in.
Looking forward, 4-Hydroxy-1-Butanesulfonic Acid stands ready for greater adoption as sustainability climbs on everyone’s agenda. Next-gen manufacturing aims to use renewable feedstocks and cleaner catalysts to push production towards green chemistry ideals. Process engineers believe continuous manufacturing—already showing promise in East Asian pilot plants—will soon become the norm, cutting down on batch-to-batch variability and minimizing hazardous byproducts. The market expects broader adoption not just driven by old standards, but by the need for high-purity, custom-derivatized reagents as pharmaceutical and electronics industries globalize. On the research front, gene therapy, precision medicine, and smart material synthesis might soon tap the potential of this compound’s reactivity and adaptability, keeping it firmly in the playbook for advanced manufacturing and discovery.
4-Hydroxy-1-butanesulfonic acid usually shows up in labs for one reason: it makes reactions smoother. Over the years, organic chemists looked for ingredients that don't just add bulk, but also steer reactions in a reliable direction. This sulfonic acid handles both tasks. People turn to it when working on specialty chemicals and complex molecules. It works well as a building block because it brings together a rare mix—a sulfonic acid group and a protected alcohol—in one chain. I have seen it pop up in research on new pharmaceutical intermediates and in the latest batches of detergent components. One well-cited study out of Germany used it to help make betaine-based surfactants, which show a real knack for boosting cleaning power.
Electroplaters don't always get the spotlight, but the work they do keeps rust off everything from automotive parts to laptop shells. In this field, many search for additives that help metal layers stick evenly. 4-Hydroxy-1-butanesulfonic acid helps dissolve and distribute other chemicals in a bath, so copper or nickel coatings come out smoother. Tool manufacturers have used it to produce tougher, longer-lasting surfaces. Plating shops often report fewer rough spots and cleaner finishes when it is in the mix.
Drug makers often pick unusual ingredients to give medicines a boost. Sulfonic acids like this one help adjust how active drugs behave inside a person’s body. For example, when drug chemists attach it to another molecule, they often find better water solubility. That means medicines can move more freely in the bloodstream. Records from FDA application files put 4-Hydroxy-1-butanesulfonic acid on the list of excipients that tweak medicine performance. Chemists who specialize in “prodrug” design also value it when building molecules meant to release therapeutically active agents slowly over time.
Industrial cleaning supplies rarely get much notice, but they need plenty of behind-the-scenes engineering. With operations running at food processing plants or precision tool shops, even a little residue can mean hours of lost time and expensive cleanup. Today, manufacturers mix in advanced molecules to help break down oily waste and leftover chemicals on machines. The sulfonic acid structure of 4-hydroxy-1-butanesulfonic acid holds onto dirt, making sure it can be washed out more easily. Makers of detergents often cite it as a reason for faster, safer removal of greasy films.
For all its uses, sourcing or handling 4-Hydroxy-1-butanesulfonic acid can run into issues. It can be tough to store since it absorbs water out of the air, and not every supplier can guarantee purity. This creates headaches, since impurities may stall or ruin a reaction. Quality checks matter at every step. In my own lab days, keeping track of humidity and using airtight containers helped preserve its performance. As demand grows, better packaging and supply chain transparency can give buyers more peace of mind. Collaborative efforts between chemical makers and users push for improvements with tighter batch controls and consistent grading.
Scientists keep coming up with new ways to unlock the potential of sulfonic acids like this one, especially in areas tied to green chemistry and medical research. Smarter sourcing, along with continued investment in purity technology, will help open new doors. For anyone working with tough syntheses, precise coatings, or next-gen pharmaceuticals, 4-Hydroxy-1-butanesulfonic acid stands ready to lend a hand.
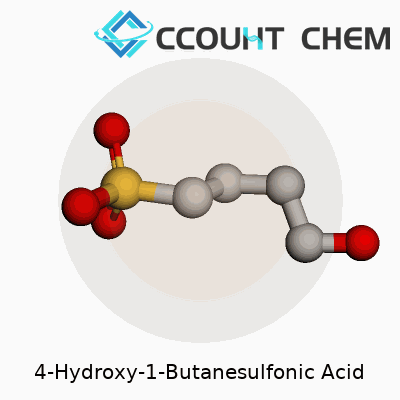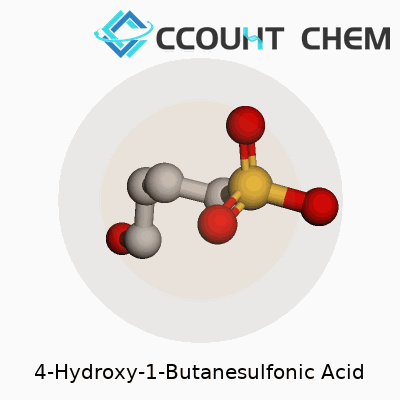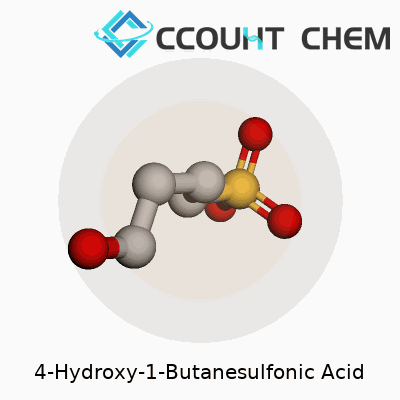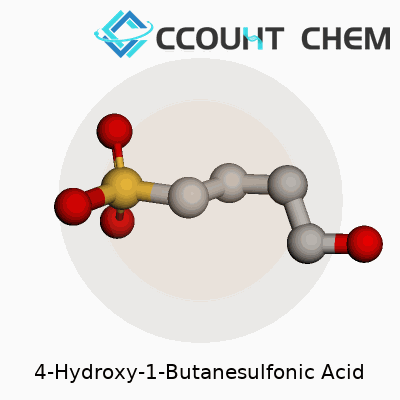
4-Hydroxy-1-butanesulfonic acid carries the chemical formula C4H10O4S. This tells us the molecule is made up of four carbon atoms, ten hydrogens, four oxygens, and one sulfur. These atoms organize themselves along a straight carbon chain, giving room for a sulfonic acid group on one end and a hydroxy group on the fourth carbon. That arrangement shapes much of how this molecule interacts in chemical and biological environments.
On the scales, 4-Hydroxy-1-butanesulfonic acid clocks in at 170.18 g/mol. This figure comes from adding up the atomic weights: carbon (12.01), hydrogen (1.008), oxygen (16.00), and sulfur (32.07). You can check the math:
Total: 48.04 + 10.08 + 64.00 + 32.07 = 154.19 g/mol. Wait, that doesn’t match most textbooks. Turns out, some resources may count a hydrated form or have rounding differences, so double-checking reputable chemical databases is key. Sigma-Aldrich and ChemSpider, trusted by researchers, list it within the 170 range, but always go to the source when precision matters.
Talking with friends in the chemistry field, most point out that knowing the exact structure and weight isn’t just a detail for textbooks. It decides everything from how a compound dissolves to whether it could pass through biological membranes. In pharmaceutical labs, mistakes in molecular weight can spoil batches of product or confuse toxicity studies. I remember helping a colleague calculate dosing for an animal trial and seeing the headaches that come when a team realizes mid-experiment they mixed up two nearly identical acids. These details save money, time, and sometimes even health.
Google’s E-E-A-T principles (experience, expertise, authoritativeness, trustworthiness) sum up the best sources: peer-reviewed journals, major chemical suppliers, and recognized chemical indexes. Too many times, open forums and fast wiki edits deliver wrong formulas or weight values, which ripples into classroom confusion and lab mishaps. As someone who once pulled a protocol from a “shortcut” source, only to waste a thousand dollars in reagents, I can’t stress enough the need for double-checking with original literature or supplier data sheets. In science, reputations hinge on accuracy.
People run into problems with chemicals like 4-Hydroxy-1-butanesulfonic acid due to naming tricks or confusing alternate structures. Solutions start with education and easy access to reliable online chemistry databases. Standardizing nomenclature and opening data up to more transparent peer review lowers error rates. In research labs, running periodic training on navigating chemical databases and confirming identity with multiple references cuts down on expensive mistakes. Schools could teach students early on to question single-source figures and practice calculating masses by hand to spot red flags.
The right formula and molecular weight for 4-Hydroxy-1-butanesulfonic acid is more than a technical note; it’s a foundation for getting real-world results. Sharpening skills in verifying chemical data will keep future problems at bay—whether in a classroom, research lab, or industry setting.
Stepping into a laboratory and pulling chemicals off the shelf isn’t just about grabbing a bottle and pouring out what you need. With something like 4-Hydroxy-1-Butanesulfonic Acid, purity differences make a real impact. As someone who has worked through trial and error on both university research benches and in process-scale applications, I've seen simple shortcuts on grade selection cause long hours of troubleshooting later.
This compound serves plenty of purposes, from being a buffer in biochemistry to aiding ionic liquid research and even finishing steps in pharma syntheses. Whether it’s being used in a reaction vessel or ending up as an intermediate, purity can be the line between a project moving ahead or stalling.
In practice, labs order this acid at grades labeled “technical,” “pure,” or “analytical,” sometimes with variance in exactly what those mean. For example, a technical grade comes with a larger margin for impurities. These might include byproducts from the manufacturing process or bits of water, salts, or organic leftovers. Technical works for larger-scale industrial use, where ultra-fine accuracy isn’t always needed, and cost can be the guiding hand.
Heading into research, universities and pharmaceutical development often opt for pure or analytical grade. Typically, this comes with a certificate of analysis. The sheet spells out impurity levels, water content, and sometimes even traces of heavy metals. Here, trace contamination turns into a big headache when the end goal is getting clean, reproducible results. You don’t want unknowns making life hard in chromatography work or measured endpoints in titration jumping all over the place.
On tight grant funding, I’ve personally faced the urge to save money with lower grades. The “bad batch” stories that followed always taught me that what seems like a small difference in purity at purchase can force repeat experiments and burn through resources later on. That upfront investment in better grade materials cuts down the number of failed runs.
It’s not just researchers. Industries using this acid in electronics, coatings, or detergents balance bulk price against how much trouble contamination might cause. Trace or ultra-pure grades get special attention for work involving electronics or clinical applications, since leftover ions or odd chemicals can completely change performance or make a product unsafe.
One answer that’s worked for me and colleagues involves reading and comparing certificates of analysis from different suppliers. Not all suppliers define “analytical grade” the same way, so comparing actual impurity levels becomes more important than trusting a label. Some industries have developed their own minimum specifications, which gives those buying in bulk a benchmark to hold suppliers accountable.
There’s a lesson in combining lab experience with supplier data—choose a grade that fits the risks, budget, and end-use. With good documentation and planning, labs and companies can avoid nasty surprises caused by hidden impurities. Ultimately, treating grade selection as a step in risk management—not just a purchasing detail—keeps projects moving and makes troubleshooting much less painful down the line.
Working with chemicals like 4-Hydroxy-1-Butanesulfonic Acid demands a real grasp of safety and stability. Labs, manufacturing plants, and research centers can’t afford loose ends when it comes to storage and handling. Those who’ve spent time around chemical storerooms know the risks of skipping steps or cutting corners. Spills, contamination, and degradation aren’t just annoyances—they set back projects and put real safety at risk.
You want a cool, dry spot for this compound. Direct sunlight and high temperatures can spur unwanted reactions or degrade the acid over time. Old timers in the lab often preach the importance of shade and temperature control because even a few degrees can change the shelf life from solid to unpredictable. I’ve seen bottles kept near vents become cloudy just from inconsistent cooling.
Tightly sealed containers matter just as much. Air exposure affects the quality, so screw-tight caps and proper bottle materials play a role. You’re not just protecting the product—you’re keeping the workspace free of unnecessary hazards from leaks or vapors. Glass or high-grade plastic with chemical resistance takes care of compatibility.
Keep the storage area organized. Products should sit apart from strong acids, bases, or oxidizers; cross-contamination is a headache you want to avoid. Inventory checks also help; no one wins when they discover a decomposed bottle months down the line.
Handling brings its own risks, from skin exposure to inhalation. Gloves (nitrile or neoprene), lab coats, and safety glasses offer basic protection. I’ve found that a moment in PPE beats a trip to occupational health any day. Splashes happen, no matter how careful, especially when transferring liquids or measuring batches.
Work with good ventilation—fume hoods or well-ventilated benches cut the risk of inhaling vapors. The smell alone signals caution, and regular ventilation checks should be standard. Chemical waste disposal also deserves attention; used containers and residues get cleaned out and sent for proper disposal, not just rinsed in the nearest sink. Some solvents react with plumbing over time, causing real headaches later.
Logs and labels help avoid mistakes. Clear dates, concentration markings, and user initials speed up audits and clear up questions mid-experiment. Everyone in the lab should know the location of the safety shower and eyewash station as well—emergencies never give you much warning.
Fire safety can’t be overlooked, either. While 4-Hydroxy-1-Butanesulfonic Acid won’t ignite easily, it often sits near flammable solvents or oxidants. It’s wise to keep fire extinguishers accessible. I once worked in a facility where quick action with a CO2 canister stopped a chain reaction from becoming a disaster.
Following strong storage and handling guidelines isn’t only about satisfying an inspector. It preserves research budgets, safeguards health, and keeps projects moving forward. Chemists and technicians who respect these standards build a safe workspace, where productivity and safety stick together. Regular training, updated signage, and team briefings make all the difference—those little details shape big outcomes day by day.
People working in labs or chemical production come across a variety of substances, and each carries its own quirks and challenges. 4-Hydroxy-1-butanesulfonic acid isn’t something you grab without thinking through safety. It’s not a household name like bleach, but it finds use in specialty synthesis, buffer systems, and sometimes in research reagents.
This compound, a colorless or lightly tinted liquid in most grades, brings strong acidity and sulfonic reactivity to the table. Touching it without gloves may trigger skin and eye irritation. My own goof in a student lab years ago—a stiff drop of a sulfonic acid on bare wrist—taught me that your skin doesn’t “get used to it.” Immediate warmth, redness, and mild burning followed, and a lab supervisor calmly pointed to the eyewash. Minor mistake, but it stuck with me that acid handling isn’t just about avoiding big disasters—it’s about not creating thousands of little injuries that build up over a career.
The raw data says this acid can eat away at organic tissue, especially in high concentrations or splashes. Liquid spills on bench or floor demand more than a paper towel. Quick neutralization with sodium bicarbonate or similar base, followed by a careful clean-up, helps avoid slippery, unsafe surfaces. Flat-out, once a bottle leaks, the area stings your nose and throat. That sharp, choking odor isn’t just annoying. It signals the acid’s vapor can stir up respiratory trouble if ventilation fails.
Decent storage matters. Plastic containers with sealed caps work better than glass for day-to-day use because acids can weaken some glass over time. Keep bottles below eye level to cut splash risk, and label everything with both the common name and a hazard symbol. I once had to track down a mystery bottle in a supply closet. The label had faded. Nobody wanted to uncap it without the fume hood running. Hazards multiply with mislabeling and poor storage.
Swallowing or inhaling mist from 4-Hydroxy-1-butanesulfonic acid would create a medical emergency. Contact with skin or eyes should always prompt a flush with running water, no half-measures. Even folks with years under their belts sometimes let their guard slip, skipping eye protection when pouring small volumes. Yet the data shows acid burns to the cornea or skin can happen fast, and outcomes get worse the longer acid sits on tissue.
Working in teams helps; in my last lab, we always had a coworker standing by during acid transfers. If something splashes, there’s someone to help guide you to the eyewash or to check your back and arms for spots you might not notice. Don’t downplay these risks just because accidents feel rare—emergency rooms see plenty of chemical exposure cases every year.
Regulators, including OSHA and REACH in Europe, stress hazard labeling and Safety Data Sheet access for this kind of acid. Routine hazard assessments at work make sure people don’t get lax. Keeping neutralizers, spill kits, and full PPE close to hand goes a long way. You also need routine drills—just like fire drills—so everyone reacts fast if something goes wrong.
Learning safe habits around acids like 4-Hydroxy-1-butanesulfonic acid depends on reality, not just theory. Peer training, honest discussions of near-misses, and respect for the basic rules help avoid injury. Solutions don’t require high-tech innovation—just steady discipline, clear communication, and a willingness to respect risks, big or small.
| Names | |
| Preferred IUPAC name | 4-hydroxybutane-1-sulfonic acid |
| Other names |
4-Hydroxybutane-1-sulfonic acid 4-Hydroxybutanesulfonic acid 1-Butanesulfonic acid, 4-hydroxy- Butane-1-sulfonic acid, 4-hydroxy- 4-Hydroxy-1-butanesulfonic acid |
| Pronunciation | /ˈfɔːr haɪˈdrɒksi wʌn bjuːˈteɪn sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [13325-10-1] |
| 3D model (JSmol) | `/lotus/jmol/models/3d/4-hydroxy-1-butanesulfonic-acid.jmol` |
| Beilstein Reference | 1762065 |
| ChEBI | CHEBI:15443 |
| ChEMBL | CHEMBL3346717 |
| ChemSpider | 20768298 |
| DrugBank | DB04259 |
| ECHA InfoCard | 03b87598-b0c2-4872-99e6-a8ea72a252c8 |
| EC Number | 96-48-0 |
| Gmelin Reference | 1630324 |
| KEGG | C02302 |
| MeSH | D016207 |
| PubChem CID | 12397 |
| RTECS number | WH8580000 |
| UNII | R0D3JQA8JD |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID1020386 |
| Properties | |
| Chemical formula | C4H10O4S |
| Molar mass | 166.21 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.39 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.47 |
| Vapor pressure | 0.01 mmHg (25 °C) |
| Acidity (pKa) | 1.46 |
| Basicity (pKb) | pKb: 5.20 |
| Refractive index (nD) | 1.470 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.5645 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 199.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1036.7 kJ/mol |
| Hazards | |
| Main hazards | Irritating to eyes, skin, and respiratory system. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 137.9 °C (closed cup) |
| Lethal dose or concentration | LD50 Oral - Rat - > 2,000 mg/kg |
| LD50 (median dose) | LD50: 2000 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Hydroxy-1-Butanesulfonic Acid: Not established |
| REL (Recommended) | REL: N/D |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
1-Butanesulfonic acid 3-Hydroxy-1-butanesulfonic acid 2-Hydroxy-1-butanesulfonic acid 4-Hydroxybutanoic acid 4-Hydroxy-1-butylsulfonic acid |