4-Anilinesulfonic acid did not just appear in the toolkit of chemists overnight. Early dye chemists in the late nineteenth century spent years investigating aromatic amines and sulfonic acids because these molecules helped push the boundaries of synthetic dyes. As demand for vibrant, colorfast textiles exploded, factories in Europe leaned on aniline dyes and their derivatives. Curious researchers blended the chemistry of aniline and sulfonation, tracking every new color. Production of anilinesulfonic acid quickly became routine at chemical plants. That shift made new applications possible in both color chemistry and pharmaceuticals right into the twentieth century.
4-Anilinesulfonic acid, sometimes called sulfanilic acid, rests solidly within the dye intermediate family. Its single benzene ring—modified by both an amino group and a sulfonic acid—offers two strong handles for further chemical reactions. As a white to grayish crystalline solid, people working with it find it reliable in both appearance and handling. Manufacturers continue to rely on it as both a core material for azo dye production and as a versatile intermediate for other chemical syntheses. Its reach extends into analytical labs, as it helps in nitrate determination and takes on a role in several pharmaceutical syntheses.
4-Anilinesulfonic acid presents itself as a powder or crystalline solid, easily dissolvable in hot water but far less so at room temperature. The melting point generally sits above 288°C with slow decomposition, reflecting both its thermal stability and resistance to simple breakdown. With a molecular formula of C6H7NO3S and a molecular weight of about 173.19 g/mol, it manages a balance between molecular complexity and ease of measurement. Its amino group gives it basicity, while the sulfonic group ensures strong acidic characteristics. Together, these groups enable tautomerism and salt formation—a fact which has marked importance in industrial purification and dye production.
Chemists who source this compound pay attention to purity and trace contaminants. Reputable suppliers claim purity levels above 99%, with low limits on water content, heavy metals, and residual solvents. Material safety data sheets and proper hazard labeling shed light on both environmental and human risks. Hazards generally link to its ability to cause irritation given skin contact or inhalation. Lab containers display hazard pictograms, precautionary statements, and UN number information where transport regulations demand clarity. Compliance with standards such as GHS labeling brings both transparency and consistency to chemical handling.
Sulfanilic acid production does not hide behind secret techniques. The process begins with aniline as the main feedstock and concentrated sulfuric acid as the sulfonating agent. Workers carefully heat aniline in strong sulfuric acid, managing temperature to avoid carbonization or charring. Once the reaction subsides, cooling prompts crystallization of the product. Washing, filtration, and drying stepwise remove residual acid and impurities. This classic route, involving direct sulfonation, keeps the cost low and operational knowledge high—a major reason for its large-scale availability worldwide.
Chemists prize the reactivity of both the amino and sulfonic acid groups on this molecule. Diazotization of the amino group opens doors to azo dye synthesis, which forms the backbone of textile coloration. The sulfonic acid can be neutralized or replaced, allowing generation of salts or further derivatives. Researchers have experimented with coupling sulfanilic acid with various phenols and aromatic amines, each combination producing unique dye shades or functional materials. Its stable structure withstands mild oxidization, yet strong oxidants can break the ring down, providing both durability and a clear path for environmentally responsible disposal.
Beyond “4-Anilinesulfonic acid,” this compound appears in literature as sulfanilic acid, p-aminobenzenesulfonic acid, or para-anilinesulfonic acid. Marketed goods may reference derivatives or simply list its CAS number. Regulatory documents keep synonyms tight, but trade names sometimes stray for branding. To avoid mistakes, lab workers often cross-verify with structural drawings and molecular formulae.
Safe handling of 4-anilinesulfonic acid requires attention and respect for both the material and the workers. Protective gloves, goggles, and fume hoods stand between lab technicians and unnecessary exposure. Good ventilation controls the risk of airborne dust that could irritate respiratory systems. Best practice dictates immediate clean-up of spills, storage in tightly sealed containers, and careful segregation from incompatible substances such as strong oxidizers and acids. Staff training covers both the acute health risks from contact and the longer-term hazards that come from repeated exposure, tying everyday operational standards to broader workplace safety responsibilities.
Azo dye synthesis remains the most visible use for this compound, especially where large-batch coloring agents for textiles and paper are needed. Analytical chemists employ it in the Griess reaction, detecting trace nitrates and nitrites in water and food supplies. The pharmaceutical industry leverages derivatives for antibiotic research and as intermediates in drug manufacturing. Water treatment facilities sometimes test for it as a process control marker, a quiet role that marks its presence in municipal infrastructure.
R&D teams continue probing for new ways to handle and modify 4-anilinesulfonic acid. Ongoing projects seek greener pathways for both its synthesis and its breakdown. Analytical chemists look for more sensitive, selective reactions using this molecule as a cost-effective standard. Process engineers explore catalysts that boost yield and cut down on waste. Some researchers tinker with enzymatic routes for both synthesis and degradation, tying academic studies to practical improvements in both cost and environmental impact. Companies surveying new dye families still look back to sulfanilic acid as a key starting point, confirming its place in the next generation of specialty chemicals.
Recent studies highlight its relatively low acute toxicity, though chronic exposure paths raise concerns. Prolonged skin or respiratory contact can lead to irritation, so regulatory agencies stress limiting routine contact. Environmental fate studies track its breakdown in wastewater, with results pointing to the importance of controlled handling and treatment before discharge. Several papers dig into its mutagenicity profile, focusing on azo dye metabolites rather than the parent compound. Data support the notion that, with proper PPE and spill management, health risks stay manageable—making it crucial to keep workplace standards updated and obeyed.
Future-facing chemists fix their eyes on sustainable production and advanced functionalization routes for 4-anilinesulfonic acid. Industry demand endures, but expectations lean toward methods that minimize energy input and hazardous by-products. R&D heads imagine catalytic, solvent-free, or bio-based options taking center stage. Emerging applications—like materials for electrochemical sensors, water purification, or specialty pigments—could broaden its utility well beyond its original 1800s dye history. New regulations push manufacturers to rethink waste management and life-cycle assessments. As calls for safer, sustainable chemistry grow louder, sulfanilic acid stands to benefit from smart advances, blending tradition with tomorrow's priorities.
An aromatic compound with a sulfonic acid group, 4-Anilinesulfonic Acid holds a quiet yet important place in manufacturing and science. I spent time working in a research-focused chemical supply company, watching basic chemicals like this one form the backbone of dyes, pharmaceuticals, and more. With a structure based on aniline, but with a sulfonic acid group at the para position, its chemistry opens doors in multiple directions.
Ask anyone who’s handled textile dyeing how tough it is to achieve reliable, lasting colors. 4-Anilinesulfonic Acid often finds its way into the synthesis of azo dyes. These dyes bring vibrant shades to everything from cotton shirts to commercial carpets. The sulfonic acid function increases water solubility, so colors mix easier into dye baths and don’t wash out so quickly. Large dye houses source chemicals like this for their trusted performance, making sure the orange on a soccer jersey is just as bright after ten washes.
This molecule pulls double duty in pharmaceutical labs. I’ve seen it take a spot as an intermediate during the creation of drugs where a sulfonic acid group helps alter water solubility and absorption inside the body. The base aniline group acts as a solid foundation for building more complex molecules, and these tweaks make a clear difference in whether a medicine works well or leaves too much residue in the body. For some antibiotics and anti-inflammatory drugs, 4-Anilinesulfonic Acid forms a key step, often getting swapped for other groups later in the synthesis but providing exactly the right chemistry to get there.
Manufacturers don’t get a pass when it comes to safety—this is a chemical with real risks. 4-Anilinesulfonic Acid is considered hazardous to aquatic life, so responsible handling is crucial. Teams working with it wear gloves, keep eye protection on, and track every batch closely. Waste treatment specialists pay close attention and use neutralization tanks and charcoal filtration to capture any stray molecules before water heads back to rivers and lakes. I’ve watched operations get shut down or heavily fined for skipping these steps—cleaner practices aren’t just good for the planet, they keep companies out of courtrooms.
Chemists keep searching for milder, greener dyes and intermediates. Many labs invest in bench-scale tests using plant-based or less persistent alternatives. As demand rises for non-toxic consumer products, old standbys like 4-Anilinesulfonic Acid are getting a new look. Some companies switch to processes that use fewer sulfonic compounds, or blend traditional chemistry with green catalysts. The switch isn’t fast or easy, since supply chains for this chemical are deeply rooted. Still, the steady rise in consumer awareness and government regulations push industry leaders to consider smarter, safer options.
4-Anilinesulfonic Acid keeps many types of production moving, from colorfast clothes to vital medications. At the same time, it reminds us that even quiet, background chemicals need thoughtful handling. Whether in a dye shop or a big pharma plant, staying mindful of both function and impact turns good manufacturing into responsible science.
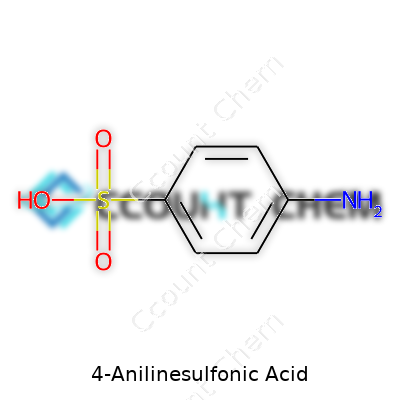
People who spend time in labs or chemical supply rooms see names like “4-Anilinesulfonic Acid” and sometimes just shrug. For someone in the dye industry, a classroom, or a specialty manufacturing plant, that name actually packs a punch. The stuff’s formula is C6H4(SO3H)(NH2), sometimes written more simply as C6H7NO3S. That tells you there’s a benzene ring with two groups hanging off: a sulfonic acid and an amine. The location of those groups—the “4-” part—matters. It means the two are opposite each other on the ring. Plenty of folks outside a chemistry circle never think twice about those numbers. Turns out, that simple “para” arrangement decides how this compound behaves in the real world.
Some people grow up thinking the only thing that matters about dyes is whether they’re vibrant or durable. Yet most consumer textiles, inks, and paper products rely on the chemistry of compounds like 4-Anilinesulfonic Acid. In the color business, workers use this compound as a building block for azo dyes—those familiar yellow, orange, and red shades in everything from shirts to markers. Chemical manufacturers learned decades ago that lining up an amine and a sulfonic acid on a benzene ring creates a unique versatility. The amine group takes part in coupling reactions while the sulfonic acid brings water solubility. Together, they keep dyes bold and long-lasting, even through a tough wash cycle.
Back in college, I remember mixing up similar chemicals and worrying about little more than spilled solutions or the occasional sniffle from a strong odor. With experience comes a new respect for safety and long-term health risks. Anilines, in general, have links to toxicity, so safe handling isn’t optional. Workers involved in large-scale synthesis, storage, or transportation need adequate protective gear and solid training. A few decades ago, stories of hazardous exposure weren’t rare, especially in places where safety rules lagged behind production targets. Today, companies ramp up ventilation systems and personal protective equipment, spurred by lessons learned and stricter regulations. The European Union, for example, places tight restrictions on how much aniline-based residue can end up in the water stream. Environmental and occupational health concerns keep driving research for cleaner synthesis and better waste management, not just for 4-Anilinesulfonic Acid but for its cousins, too.
Advances in green chemistry offer a promising road ahead. Synthetic pathways keep getting refined to cut down on hazardous byproducts. Some chemists work with enzymes or clever catalysts to make the same compound with less waste. Factories shift toward closed systems to capture and recycle water used during dye production. In my own work, it feels good to know that opportunities for improvement don’t have to mean trading away job security or profitability. It’s about smart process choices and accountability at every step—from mine to market shelf.
If the next generation understands both the formula and its global impact, we all win. Nobody wants a world where the quest for color costs rivers, workers, or communities their health. College courses should cover more than textbook reactions; they should ask tough questions about responsible production and safe use. The formula C6H7NO3S isn’t just a puzzle for an exam. It’s a snapshot of what happens when chemistry meets design, economics, and environmental stewardship—all in a single molecule.
Chemicals like 4-Anilinesulfonic acid don't spark daily conversations, but for those working in labs or manufacturing plants, the effects of handling such substances are real. Over the years, I've seen accidents happen because someone underestimated a compound they thought was pretty harmless on paper. It's a wake-up call every time.
Industry uses 4-Anilinesulfonic acid in dyes, pigments, and pharmaceuticals. The substance appears as an off-white to light brown powder, dissolves well in water, and reacts with strong oxidizers. Sometimes, it slips under the radar because it doesn't look threatening or have that pungent smell some chemicals do.
Direct contact burns the skin and eyes. People report redness, pain, and serious irritation after even minor spills. Any inhalation of dust can leave you coughing or feeling short of breath, probably from the irritation to the lining of your airways. Swallowing it by mistake leads to nausea, vomiting, and in severe cases, more serious damage to internal organs, especially the liver and kidneys.
No official cancer warning, but the data on long-term exposure is not strong. The concern comes from aniline-related compounds, some of which harm human health over time, so researchers keep a close eye on new findings. The European Chemicals Agency and US Environmental Protection Agency urge caution just because the family of chemicals includes other well-known toxic substances.
This stuff gets into waterways if waste handling doesn't follow strict rules. Fish and smaller aquatic life suffer quickly; many sulfonic compounds persist, breaking down slowly. I spent a summer volunteering along a river downstream from an old textile plant and locals always talked about the years when nothing would grow or swim there. Nature remembers, even if regulators move on.
Forget working barehanded. Nitrile gloves, sturdy goggles, and a coat keep most incidents from turning into medical emergencies. Good ventilation saves more than a few headaches for anyone handling this powder. I once spent a shift in a poorly-ventilated storeroom and left with a sore throat and watering eyes just because one barrel cracked its seal.
Disposal always trips people up. It doesn't go down the drain. Special containers and licensed waste management keep it away from regular landfill or water treatment facilities. Labs invest real money to train staff on handling these risks, and for good reason.
Engineering controls like closed transfer systems and dust extraction mean no one needs to rely on luck. Spills need containment kits at the ready, not hidden behind clutter. Companies that set up routine hazard reviews and listen to worker reports cut down on injuries by a huge margin. On the regulatory side, requiring up-to-date labeling and material safety data sheets makes a big difference for new employees and veterans alike.
Raising awareness isn't about scaring anyone away from common chemicals; it's about learning from mistakes and using facts to back up every protocol. Products we take for granted often trace back to acids and dyes that act as silent hazards. The least we can do is respect the risks and keep our guard up, whether in a university lab or an industrial warehouse.
4-Anilinesulfonic acid gets used in different manufacturing steps, especially for dyes and specialty chemicals. The compound looks like a white to off-white solid and usually arrives in well-sealed drums or bags. Its chemical structure—with both an amine and sulfonic acid group—carries real implications for how workers and lab managers should approach storage. Anyone who has spent time in a chemistry lab knows that overlooking small details in housekeeping can lead to bigger problems down the line.
Keeping moisture out makes all the difference with 4-anilinesulfonic acid. Humidity encourages clumping or even partial dissolution, and trying to weigh out sticky chemicals drags down productivity. Storing the material in a cool, dry place keeps the powder flowing freely. I remember one job at a research facility where we used silica gel packets in our supply cabinets; this low-tech trick made a clear difference, cutting waste and saving money in the long haul.
The container itself should be tightly closed, preferably in a container made of a material that stands up to strong acids. Too often, people think any plastic bag will do, but strong acids can sometimes react with or permeate ordinary plastics. A sturdy polyethylene or glass vessel holds up best. This is not a place to cut corners. Ensuring the lid fits tightly and checking seals or gaskets can stop minor leaks from becoming big headaches. Shelving should be secure and clearly labeled. Labels do not just serve compliance—they keep everyone on the same page, prevent mix-ups, and make audits less stressful.
Mixing chemicals can bring out their nasty side in a hurry. Keeping 4-anilinesulfonic acid well separated from strong oxidizers or bases reduces accident risk. In my own experience, proper storage keeps cleanups to a minimum. If you put acids next to caustic materials, sooner or later a spill or accidental mix will force everyone to scramble. Grouping chemicals by hazard class works. This makes grab-and-go work easier and cuts down on confusion when new staff join the team.
Heat speeds up chemical reactions, so stashing 4-anilinesulfonic acid away from hot pipes, direct sunlight, or furnaces keeps things safer. Most labs do fine storing it at room temperature, around 20 to 25°C, but if your workspace heats up, find cooler storage or add temperature alarms. A basic thermometer on the shelf gives early warning before problems build up. If you have ever cleaned up after a shelf of old powders has caked, you know a dry, temperature-stable spot matters more than people admit.
It’s easy to take safety drills for granted, but having a clear plan pays off. Spill kits with compatible absorbents and practical written steps for cleanup help workers stay calm if things go wrong. Eye wash stations and emergency showers should be checked regularly. Companies that maintain strong training on chemical storage see fewer incidents. I have seen shifts with good training handle minor spills smoothly while others fall apart over simple mistakes. Investing in real-world drills makes daily tasks run more smoothly and builds worker confidence.
Careful storage protects people and preserves product value. Humidity control, the right containers, separation from incompatible substances, and solid emergency prep all contribute. Attention to these basics supports both safety and quality. Small investments in training and equipment pay off every day, supporting teams and keeping operations steady.
4-Anilinesulfonic acid, also known as sulfanilic acid, doesn’t grow on trees. You make it by sulfonating aniline. That involves mixing aniline with concentrated sulfuric acid, heating it, and watching a white, crystalline product form as things cool down. The whole thing feels a lot like baking, except you trade cookies for a crucial compound in dyes, drugs, and food colorants.
In the lab, the basics sound straightforward. You pour concentrated sulfuric acid into aniline. Heat the mix to about 180°C. If you watch the temperature, handle the acid with respect, and keep your ventilation on, you get a solid product after pouring the mix into cold water and letting it crystallize. There’s no magic—just solid, traditional chemistry.
Synthetic dyes changed the world’s wardrobes. Much of that started with simple building blocks like 4-anilinesulfonic acid. This compound helps make azo dyes, which color everything from textiles to printer ink. I remember visiting a fabric dyeing unit as a student and seeing pale powders, like this acid, getting ready to bloom into color in vast vats. These powders aren’t just raw materials—they’re silent drivers behind whole industries.
Modern medicine taps into the same chemistry. Sulfa drugs, among the first antibiotics, rely on sulfanilic acid as a foundation. Despite all our advances with newer drugs, the original sulfamethoxazole combo antibiotics are still part of the World Health Organization’s essential medicines list.
Sulfonation isn’t just a classroom demo. The heat and acid required can burn skin and cause lung trouble if fumes escape. In real factories, even modest spills carry danger. Controlling temperature prevents runaway reactions, where too much heat can make a bad day worse fast. Real people—technicians and engineers—stand just a glove’s thickness from these risks every time they start up a reactor.
Then there’s waste. Once the main reaction finishes, rinsing the product leaves behind acidic and aromatic rinse water. Historically, too many plants dumped this directly into rivers, leaving fish gasping. Today, environmental rules force better choices. Neutralizing waste acid, recycling water, and capturing leftover organics make for cleaner air and rivers, and just seem like common sense when you live nearby.
Old factories stick with sulfuric acid, heat, and plenty of water. The best improvements keep workers safe and cut pollution. Automated reactors keep hands away from corrosive acid. Modern plants recover and reuse sulfuric acid, reducing demand for new acid and cut costs at the same time—something any production manager would cheer.
Researchers hunt for even cleaner paths. Some explore catalysts that let the reaction work at lower temperatures or use milder conditions. Others run reactions in water instead of harsher solvents, keeping fumes down. Roadblocks pop up, like finding a catalyst that works without killing yields or driving up costs. But progress rarely comes easy in industrial chemistry.
It’s easy to take the color in clothes or safe, reliable medicines for granted. These simple molecules, like 4-anilinesulfonic acid, underpin much of daily life. Each batch that’s greener and safer helps clean up the air, the water, and the lives of people who run the plants. That seems worth the effort—whether you’re a seasoned chemist, a plant worker, or just a consumer with an eye for bright colors and good health.
| Names | |
| Preferred IUPAC name | 4-(Phenylamino)benzenesulfonic acid |
| Other names |
Sulfanilic acid 4-Aminobenzenesulfonic acid p-Aminobenzenesulfonic acid p-Anilinesulfonic acid |
| Pronunciation | /ˈæn.ɪ.liːnˈsʌl.fə.nɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 88-21-1 |
| Beilstein Reference | 79587 |
| ChEBI | CHEBI:9476 |
| ChEMBL | CHEMBL274787 |
| ChemSpider | 12275 |
| DrugBank | DB13960 |
| ECHA InfoCard | 100.010.030 |
| EC Number | 213-679-9 |
| Gmelin Reference | 1592 |
| KEGG | C07079 |
| MeSH | D000881 |
| PubChem CID | 8781 |
| RTECS number | BO5075000 |
| UNII | 1F7TY29KM8 |
| UN number | UN2586 |
| CompTox Dashboard (EPA) | DTXSID1030937 |
| Properties | |
| Chemical formula | C6H7NO3S |
| Molar mass | 215.24 g/mol |
| Appearance | White to light brown crystalline powder. |
| Odor | Odorless |
| Density | 1.31 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -0.8 |
| Acidity (pKa) | -0.7 |
| Basicity (pKb) | 6.41 |
| Magnetic susceptibility (χ) | -5.4 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.654 |
| Dipole moment | 2.37 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -255.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1606 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergic skin reaction |
| GHS labelling | GHS05, GHS07 |
| Pictograms | OC1=CC=C(C=C1)NS(=O)(=O)O |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 3-1-1 |
| Flash point | > 230 °C |
| Autoignition temperature | 615°C |
| Lethal dose or concentration | LD50 (oral, rat): 750 mg/kg |
| LD50 (median dose) | LD50 (median dose): > 2000 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | Rel. 1.0 |
| Related compounds | |
| Related compounds |
Aniline Sulfanilic acid Sulfanilamide p-Toluenesulfonic acid Benzenesulfonic acid 4-Aminobenzenesulfonic acid 4-Nitroaniline Sulfanilic acid sodium salt |