Interest around 4-Aminotoluene-3-sulphonic acid dates back to the growth of the synthetic dye industry in the late 19th and early 20th centuries. Companies and chemists needed intermediates that offered both flexibility and reliability, especially for colors that stuck fast through washes and sunlight. Laboratories moved beyond naturally sourced aniline dyes, seeking compounds that fattened the industrial pipeline and made things cheaper, brighter, and longer-lasting. People once depended on slow, uncertain extraction from scarce plant or animal origins, but public appetite for colored cloth and vivid products demanded something better. 4-Aminotoluene-3-sulphonic acid turned out to be a workhorse here—easy to tweak, forgiving in the pot, and eager to form the backbone of a dozen other chemicals. Its emergence signaled a shift from artisanal chemistry to real industrial muscle.
4-Aminotoluene-3-sulphonic acid shows up mostly as a fine powder or crystalline solid, clean and off-white, sometimes blushing pinkish if handled wrong or exposed to air for too long. As an intermediate, it gets pumped into all sorts of syntheses—showing its face for a moment, then transforming to feed off into something more complex. It lands on purchase orders for textile dye producers, pharmaceutical labs, pigment makers, and even the niche world of paper-processing aids. Because of its chemical backbone, it allows for varied downstream transformations, a factor that end-users value for cost-containment and broad application. Bottles and drums of this material are rarely visible to the public, but companies quietly shuffle tons every year as the foundation for brighter, more colorfast, and more specialized products.
Anyone who has handled 4-Aminotoluene-3-sulphonic acid recognizes its slightly gritty texture and its ease in blending with water. The melting point hovers between 225°C to 235°C. Once in solution, the material demonstrates its acidic nature, presenting a sulfonic acid group at one end and a stubbornly reactive amine on the other. This combination lets it behave both like a base and an acid during reactions, giving chemists the chance to fine-tune conditions and steer reactions toward complex targets. In dry form, it holds up well under typical storage conditions. It resists breaking down in air, avoids decomposition under sunlight, and doesn’t emit unpleasant odors—handy features for industrial-scale handling.
Companies ordering or handling this chemical pay close attention to the purity rating, which commonly reaches up to 98% or 99% for most synthetic work. Water content stays under 1%. The product’s packaging labels declare its molecular formula (C7H9NO3S), molecular weight (187.22 g/mol), batch numbers, production dates, and hazard warnings in line with GHS/OSHA requirements. Producers detail pH values in solution, solubility in organic solvents versus water, and any byproduct traces—amino-benzenes, sulfonic fragments—down to tenths of a percent. Mistakes or lapses in labeling crop up now and then, especially in smaller regional supply chains, opening the door for regulatory fines or product recalls. Industry-wide, the baseline today is much stricter than it was a generation ago, mostly because trace residues and cross-contamination can ripple out downstream, especially in sensitive pharmaceutical uses.
Most commercial batches of 4-Aminotoluene-3-sulphonic acid start from toluene sulfonic acid derivatives. The classic approach runs through nitration, sulfonation, then reduction. Toluene gets the sulfonic group tacked on, then the nitro group introduced. Later, metal catalysts or reducing agents generate the free amine group. Early manufacturing relied on batch reactors and slapdash temperature monitoring, which created inconsistent yields and purity problems. Over decades, improvements in reactor design, catalyst recycling, and in-line analytical monitoring streamlined the process. Plant operators today measure reactions by the second and tweak flow rates or acid concentrations in real time, making safer and more controlled output possible. Issues like exothermic surges, which used to spell disaster in older settings, are more manageable with today’s tools.
4-Aminotoluene-3-sulphonic acid stands out for what chemists call “synthetic accessibility.” Its exposed amine invites acylation, diazotization, and coupling reactions—central to making azo dyes, for instance. The sulfonic acid moiety ties the molecule down, enhancing water solubility and locking colorants onto fibers or paper. In the right hands, this material serves as a springboard for hundreds of finely tuned intermediates and final products. Over the years, researchers have played with every modification imaginable—halogenating the toluene ring, swapping in various alkyl groups, even squeezing the molecule into fused ring systems for richer color or better conductivity. Each new tweak affects stability, reactivity, or end-use profile, so every alteration brings a new round of testing.
The chemical goes by several names in the literature and on supplier catalogs: 4-Aminotoluene-3-sulphonic acid, 3-Methyl-4-aminobenzenesulfonic acid, 2-Amino-5-methylbenzenesulfonic acid, and Para-amino-toluene-meta-sulfonic acid. CAS Registry commonly logs it as 98-44-2. European and Asian suppliers throw out trade names based on the region, sometimes abbreviating to ATS or denoting a specific purity or intended market. These alternative names and numbering conventions create confusion, especially for researchers or buyers working across multiple countries, making clear communication with suppliers and careful SDS sheet checking important.
Bulk quantities come with safety data sheets referencing risks associated with eye and skin contact, especially given its acidic nature and potential for strong irritation. Industry regulations demand gloves, goggles, and in well-run plants, fume hoods or closed transfer systems. Managers looking to avoid trouble adopt the stricter side of recommended procedures. Accidental spills, while not explosive or highly toxic, can disrupt plant sewer systems because the acid group drops local pH and can affect bacteria-based wastewater treatment. Over the years, regulatory oversight from EPA, REACH, and OSHA pushed companies toward secondary containment, automated monitoring, and improved emergency response planning. Above all, safety training drills and accessible first-aid gear make the most difference, as most problems start with basic lapses in handling or care.
One of the major uses for this chemical lands in the world of azo dye manufacturing, where it forms the base for vibrant reds, oranges, and even brown shades used in textiles, plastics, and inks. Its stability and water solubility make it hard to replace in colorant systems meant for clothes that endure harsh detergent cycles and prolonged sun exposure. Beyond dyes, the pharmaceutical sector employs this acid as a step in creating anti-inflammatory drugs, sulfa antibiotics, and a range of pain medications. Not every user grabs it for final products—many warehouses stock it for onward synthesis, where its amine and sulfonic acid groups open creative routes to specialized molecules. Environmental and paper industries sometimes choose it for additives improving optical brightness or as a dispersant aid in coatings.
Researchers keep honing in on new derivatives and less hazardous processing methods. Many turn to greener synthetic pathways—aiming for water-based reactions over organic solvents. Advances in catalytic chemistry lowered costs and waste, letting labs dial down on toxic by-products. Scientists tinker with inactive analogs to open up more use in food-dye sectors or as drug intermediates with milder side effects. On the materials side, interest grows in tweaking the compound for UV-blocking films, conductive polymers, and even as a precursor for specialty nanomaterials. There’s quiet competition between academic labs and industry, each chasing a new patent or route that reduces cost, increases performance, or sidesteps regulatory headaches over toxic waste.
Researchers and regulators keep eyes on toxicity profiles across this class of compounds. Acute exposure shows mild effects—mostly skin and eye irritation, rarely causing systemic effects at low levels. Inhalation of dust can cause respiratory irritation, especially in poorly ventilated facilities. Earlier eras gave little thought to chronic impacts, but animal studies over time show low potential for mutagenicity, and very low risks for carcinogenicity compared to other aromatic amines. Environmental toxicity does flag concerns when discharge enters water systems, mostly around aquatic organisms sensitive to pH swings or the persistence of sulfonic groups. Studies over decades continue to refine safe exposure limits. Regulatory bodies update workplace standards as the evidence evolves, nudged by academic findings and industrial incident reports.
As industries move toward “greener” targets and consumers push for fewer persistent chemicals in the environment, there’s strong momentum for developing new production methods for 4-Aminotoluene-3-sulphonic acid. Green chemistry projects highlight routes that start with renewable toluene sources, recycle catalysts, and limit acidic effluent. Application expansion in electronics, solar films, and specialty plastics could drive growth, but only if toxicity and environmental data support safe, long-term use. The real change will likely come on the back of big investments in process safety and in better recycling or reclamation strategies that keep waste out of rivers and ground. For companies and researchers, every small efficiency or reduction in hazardous output represents a step not just toward profit, but public trust and long-term license to operate. As regulatory demands and consumer awareness keep rising, only those who master safety, transparency, and environmental stewardship will thrive in this new landscape.
Anyone who's cracked a chemistry textbook might remember the tongue-twister names like 4-Aminotoluene-3-sulphonic acid, but this compound does more than fill space in industrial inventories. Years ago in a dyes plant, I saw how these chemicals drive entire supply chains. This one, in particular, plays a pivotal role in producing azo dyes. These aren't just laboratory curiosities. Azo dyes become the orange in packaging, the red in textiles, the yellow in paints. No other category churns out such vibrant, long-lasting color at scale. The reason these colors withstand sunlight and multiple washes comes straight from compounds like 4-Aminotoluene-3-sulphonic acid.
Beneath the surface, factories use this acid to jump-start the chemistry that brings finished products to shelves. The amino and sulfonic groups on the molecule give it unique reactivity. Chemists love it for diazotization, a reaction that opens the door for endless possibilities—high-performance pigments, specialty dyes for food safety indicators or medical imaging. I once toured a factory southwest of Shanghai where workers converted this compound daily, feeding it into huge reactors that fill entire rooms. On site, the work can get messy, but without it, we wouldn't have many of the vivid colors lining supermarket shelves or coloring pharmaceutical coatings.
In places that manufacture aromatic sulfonic acids in bulk, safety becomes a topic that can't be skirted. The sulfonation process generates strong acids, heat, and sometimes hazardous byproducts. I remember, during a visit to a mid-sized plant in Gujarat, the direct air exhaust carried a trace of sulfur. Good ventilation and routine safety drills made all the difference. As the world pivots toward eco-conscious production, efficient absorption systems and scrubbers help keep emissions in check. These steps not only protect workers, but also communities that live close to manufacturing plants.
When major brands talk about sustainable supply chains, they often focus on end products—organic cotton, recycled polyester, biodegradable plastics. None of this works without looking at everything upstream. Substitute chemicals for textile dyes, such as nature-based alternatives, get plenty of headlines. Yet, petrochemical-based intermediates like 4-Aminotoluene-3-sulphonic acid remain crucial because current green replacements can't yet match consistency, availability, or cost. This doesn’t mean sticking with old methods forever. Tighter regulation, investment in water recycling, and new catalytic processes can reduce waste. Some companies have shifted toward closed-loop systems that recover spent chemicals on site, cutting both pollution and operational costs.
For every factory that invests in training staff on safe handling, communities gain real protection. Upgrading reactors and improving process integration isn’t just a spreadsheet exercise—it reduces spills and energy use. Open conversations between producers, regulators, and customers can push the industry to adopt cleaner, more efficient practices. Those of us working on the ground keep searching for new chemistry that balances cost, color quality, and environmental care. Day to day, that's what shapes the future of materials science—one batch of chemicals at a time.
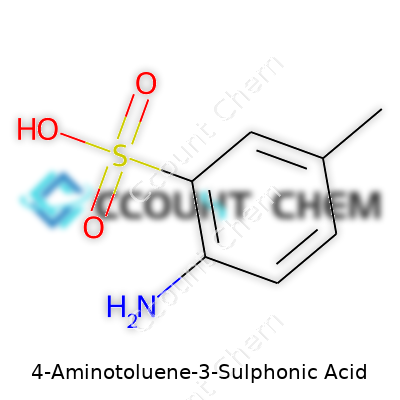
People who work with dyes or specialty chemicals run into the name 4-Aminotoluene-3-Sulphonic Acid often. Its structure means something real in the lab and in production. Chemists recognize it as a benzene ring holding an amino group, a methyl group, and a sulfonic acid group, each falling on different positions of the core ring.
The chemical formula for 4-Aminotoluene-3-Sulphonic Acid is C7H9NO3S. If you glance over that formula, you see seven carbon atoms in the backbone, a methyl group attaching itself as a single carbon, an amino group doing heavy lifting for reactivity, and a sulfonic acid group making the compound water-soluble and strong in its applications. That precise structure allows industries to predict how this molecule will behave, whether mixing with other chemicals or being transformed into dyes that wind up on textiles all around the world. That formula is not just a jumble of letters and numbers – it's a recipe for making products you'd see every day.
In the dye industry, exact molecular formulas are crucial. Trying to create consistent color shades in batches? A single slip in the formula throws off everything. People working in these factories rely on formulas like C7H9NO3S because they cut out the guesswork. Waste shrinks. Safety goes up. Product quality holds steady. Chemists studying this acid look at the positions of the amino and sulfonic acid groups: 4 and 3. This tells them how the acid reacts and attaches to other molecules during synthesis, which makes or breaks large-scale manufacturing.
Misidentification is not rare. Some might confuse similar sulfonic acids or swap the numbers, which can lead to real problems like incorrect waste handling or poor batch quality. If the molecule’s formula is off, the product fails downstream tests, and that means money and resources down the drain.
You’ll see 4-Aminotoluene-3-Sulphonic Acid used as an intermediate for creating dyes used on wool, nylon, and even some types of leather. Because the chemical dissolves easily in water due to the sulfonic acid group, it lets manufacturers apply color more evenly and reliably. Beyond dyes, the same molecule finds itself in pharmaceuticals and fine chemical synthesis, where precision means safety, efficiency, and solid results.
A common challenge stems from impurities during large-scale production. Keeping the chemical formula consistent and the product pure protects workers from unexpected reactions and cuts environmental risks. Quality control teams lean on spectroscopy and chromatography to check the formula before moving forward. Analytical chemists look for those tell-tale atomic counts: seven carbons, nine hydrogens, one nitrogen, three oxygens, and a sulfur. Getting each batch checked builds trust for downstream users.
Efforts to tighten chemical tracking and digitalize inventories make a difference. Scanning QR codes on drum labels or setting up smarter chemical management systems give staff fewer chances to pull the wrong stock. Training matters, too. Anyone working in labs or production needs to know what molecules look like—in practice, not just on paper.
In my own lab experience, cutting corners on formula verification always ended up with delays or unwanted surprises. The lesson? Stick with what you know, double-check the formula—C7H9NO3S—and focus on clear, careful documentation every step of the way.
Walking through a chemical plant or even glancing at a Material Safety Data Sheet, you'll likely come across names like 4-Aminotoluene-3-sulphonic acid. Many folks working in textile dye production or related labs grow familiar with these mouthfuls. Looking at the multi-syllabic label doesn't scream danger, but hidden risks exist behind so many industrial powders and crystals. The real question boils down to this: Will handling 4-Aminotoluene-3-sulphonic acid put your long-term health on the line?
Nobody grabs a coffee with hands still dusty from any industrial chemical, and there's good reason. This compound, used as both intermediate and dye-building block, comes with a record. Direct contact can trigger skin and eye irritation. Inhaling the fine dust sometimes leads to sneezing and nose discomfort. People unwrapping new bags without proper ventilation often share stories about mild headaches or persistent coughs. Science hasn't uncovered catastrophic consequences for brief or low-level exposure, luckily, but that doesn't give a free pass.
Worker studies show that those regularly breathing in or spilling aromatic sulfonic acids can develop chronic irritation issues. Sometimes people develop heightened sensitivity after repeated contact—kind of like those who suddenly react to latex or certain metals. Itching or rashes disrupt focus and work output, which nobody wants.
Cancer risk sits at the top of everyone's worry list. Fortunately, expert groups like the World Health Organization and US EPA haven't slapped 4-Aminotoluene-3-sulphonic acid with a known cancer-causing label. Unlike some relatives in the aromatic amine family, it lacks a legacy of pushing cells toward runaway growth. Still, it shares enough chemical cousins to keep researchers alert, especially since exposure to amines and sulfonic acids sometimes links to bladder cancer or blood cell changes in the right conditions and doses.
Thousands of tons of this compound move across the planet each year. Cast-off powders can drift onto soil or tumble down pipes into rivers. Once there, 4-Aminotoluene-3-sulphonic acid doesn't vanish overnight. It clings to water, breaking down slowly, which means it sticks around long enough for fish and other animals to come into contact. Routine tests still haven't shown dramatic toxic effects at the levels usually found outside plants, but big spills push those numbers up. Here, fish-kills and plant die-offs draw local headlines.
Real safety starts with the basics. At every plant I've visited, gloves, goggles, and dust masks sit right next to the chemical bins. Training makes or breaks safe handling. Sharing stories about people who protected themselves helps new staff understand why routines matter. Washing up before eating, not carrying work clothes home, and quick cleanup after spills cut the odds of irritation or longer-term harm.
Factories that add extra ventilation, limit staff time in dusty zones, and hold regular health checks tend to report fewer sick days. Open conversations about health symptoms lead to faster interventions, avoiding the kind of damage that sneaks up over years.
4-Aminotoluene-3-sulphonic acid doesn't sit in the same hazard class as cyanide or asbestos, but it deserves more respect than flour or table salt. Irritation isn't a small thing if it ruins concentration, disrupts daily routines, or signals bigger trouble down the road. We can't ignore how production and disposal ripple through local communities, especially near water sources. Pushing for better training and safety supplies keeps more people out of danger and supports cleaner industries. Health safety never comes from luck; it grows out of hard-earned lessons and a steady eye on the facts.
Working around chemicals like 4-Aminotoluene-3-Sulphonic Acid isn’t just a technical matter—it’s about keeping people out of harm’s way and preventing costly surprises. I’ve seen warehouses turned upside down by a chemical mishap, and poor storage tends to be the culprit. Strong protocols matter for anything with “sulphonic” in the name, but proper storage often gets overlooked.
No sealed lab is needed. 4-Aminotoluene-3-Sulphonic Acid stays stable as long as it’s kept dry and away from any heat source. Even in a small shop or school store room, humidity causes caking, and heat can speed up reactions you really don’t want.
Based on my own stints in chemical storerooms, a cool, dry environment—between 15°C and 25°C, protected from sunlight—makes sense. The chemical holds up in a sturdy, well-sealed container. Avoid glass jars with loose lids; a plastic screw-top bottle with a tight seal or a proper steel drum works better for larger volumes.
Complacency creeps in after years of sorting bags of white powder. One day I opened what I thought was sodium chloride, only to realize it wasn’t clear what was inside. Always use clear, permanent labels. Include hazard warnings right where everyone can see them. Anyone handling chemicals—whether in a classroom or a factory—must identify the contents at a glance. This single habit has kept me and my colleagues above water more times than I can remember.
Store 4-Aminotoluene-3-Sulphonic Acid away from anything it could react with. Acids and oxidizers shouldn’t share shelf space. Don’t pile up containers in an old paint closet with bleach, cleaning agents, or strong bases nearby. Mixing spills or vapors can bring on toxic clouds or fire risks. OSHA and NIOSH guidelines flag strong separation as a basic requirement—one that has saved real lives.
No storage site stays clean by accident. Every chemical storage area benefits from adequate ventilation. Fumes build up slowly, especially in poorly ventilated corners. I’ve lost count of the times simple open windows and fans prevented headaches and respiratory irritation. Efficient ventilation keeps air clear and reduces any risk of dangerous accumulation.
Spill kits deserve a dedicated spot near the storage area, not stashed away two floors down. My time in older lab settings showed how crucial that proximity is. Absorbent materials, gloves, eye protection, and dustpans should always sit within arm’s reach. Clear training, quick access, and the habit of cleaning up immediately mean minor messes never spiral out of control.
Curiosity, shortcuts, and a lack of oversight cause most storage accidents. Access should always be limited to trained personnel who understand the hazards. Lock cabinets and storerooms when unattended. Keep SDS documents handy, and revisit training anytime a new batch arrives.
Regulatory rules may feel like a hassle, but the incidents I’ve witnessed prove why fines and shutdowns happen for a reason. Compliance with standards set by OSHA, the EPA, and local authorities isn’t about ticking boxes—it’s about keeping employees, neighbors, and the environment out of trouble.
4-Aminotoluene-3-sulphonic acid shapes more than a handful of industries. The name doesn’t roll easily off the tongue, but the impacts show up in daily life and on factory floors around the globe. I’ve noticed its fingerprint across both scientific research and regular industrial process notes.
The textile and dye world uses this compound as a building block. Companies making azo dyes lean on this acid for bright, lasting colors. Without it, the colors in much of today's fabric won’t stick or shine as vibrantly. I’ve seen production labs where batches of reactive dyes demand a steady feed of 4-aminotoluene-3-sulphonic acid just to keep up with color demands from fashion and upholstery clients. Water-solubility and the stability of its chemical structure cuts down rework, which matters when time and water use both carry a price tag.
Medicines and intermediate chemicals trace part of their roots to this molecule. Pharmaceutical firms use it to stitch together key steps in drug synthesis processes. Think pain relievers or antibiotics—these complex pills often build from not-so-obvious building blocks. By connecting simple aromatics to drug candidates, chemists open doors for new treatments. For all the talk about drug pricing, efficient, reliable building blocks like this can shave real dollars off manufacturing costs.
Lab notebooks fill up with notes on this compound even beyond the big industries. Specialty chemical shops often blend it into products that tweak the performance of resins or plastics. Rubber articles, adhesives, coating additives—all draw from this feedstock. For a while, I noticed requests from R&D departments hunting for unique derivatives, hoping to land that edge in product formulations.
Wastewater treatment and pollution control firms sometimes add this acid to chemical mixtures that break down dyes or chart the fate of pollutants. Back when I worked alongside an environmental chemist, she described using it as a marker to monitor reactions that scrub out colorants from discharged water. These small moves add up, especially when communities want cleaner rivers.
No chemical story runs only on utility. Health and safety rules and a growing push for green chemistry give companies new challenges. Longtime manufacturers now invest in closed-system handling and advanced personal protective gear. I’ve seen companies cut fugitive emissions to near zero by adding better extraction and flexible storage. Europe and parts of East Asia demand high standards for import approval—those rules now ripple out to global producers, raising the bar for all.
Technology promises cheaper and greener methods for synthesizing this compound. Researchers have begun switching from traditional, high-energy routes to lower-impact pathways. If suppliers bet on these changes, downstream firms stand to benefit from both cost containment and compliance with stricter environmental laws. Training matters too; companies that train workers in the smart handling and recovery of process water see fewer accidents and lowered overall costs.
| Names | |
| Preferred IUPAC name | 4-Amino-3-methylbenzenesulfonic acid |
| Other names |
3-Sulfo-4-methylaniline 4-Amino-m-toluenesulfonic acid p-Amino-m-toluenesulfonic acid Tobias acid |
| Pronunciation | /ˈfɔːr əˈmɪnoʊ.tɒl.juːˌiːn θriː sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-44-8 |
| 3D model (JSmol) | `load =3d7g` |
| Beilstein Reference | 1211644 |
| ChEBI | CHEBI:73849 |
| ChEMBL | CHEMBL16306 |
| ChemSpider | 10201 |
| DrugBank | DB16245 |
| ECHA InfoCard | ECHA InfoCard: 100.007.606 |
| EC Number | EC 226-193-2 |
| Gmelin Reference | 79039 |
| KEGG | C06422 |
| MeSH | D014038 |
| PubChem CID | 8597 |
| RTECS number | WS5075000 |
| UNII | G9T22GI9DN |
| UN number | UN2582 |
| Properties | |
| Chemical formula | C7H9NO3S |
| Molar mass | 173.20 g/mol |
| Appearance | White to grey powder |
| Odor | odorless |
| Density | 1.31 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.2 |
| Vapor pressure | 0.000016 hPa (25 °C) |
| Acidity (pKa) | 1.45 |
| Basicity (pKb) | 6.83 |
| Magnetic susceptibility (χ) | -81.0×10⁻⁶ cm³/mol |
| Dipole moment | 4.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 236 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -672.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1448.8 kJ/mol |
| Pharmacology | |
| ATC code | ATC code not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 209.2°C |
| LD50 (median dose) | LD50 (median dose): 7000 mg/kg (rat, oral) |
| NIOSH | SL8575000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | Not established |
| Related compounds | |
| Related compounds |
Toluene-2,4-diamine p-Toluenesulfonic acid Sulfanilic acid 4-Nitrotoluene-2-sulfonic acid Metanilic acid |