Chemistry labs everywhere keep coming back to 4-(2-Hydroxyethyl)Piperazin-1-Ylethanesulphonic Acid, known as HEPES. The compound cropped up in the 1960s among Good’s buffers, part of a hunt for reliable pH buffers for biological research. Tests on cell cultures needed something stable, something that wouldn’t break down under light. HEPES has now earned its keep at countless benchtops, especially as sodium bicarbonate-based buffers grew unpredictable and CO2 incubators found wider use. The big shift from old-fashioned phosphate buffers landed HEPES in a starring role, mostly because scientists demanded gentle and stable pH control.
HEPES appears as a white crystalline powder, dissolving readily in water. No one mistakes its faint odor for much, but its ability to keep pH steady speaks volumes. Researchers like HEPES because most cells stay happy in it, even at heavier workloads. Commercial labs, academic researchers, and biopharmaceutical makers all keep stockpiles nearby, since about every experiment from enzyme studies to tissue engineering calls for something like this.
The chemical formula, C8H18N2O4S, places the molecular weight at 238.3 g/mol—handy for making mixes in the lab. The acid fits into the zwitterionic buffer group, meaning the molecule carries both positive and negative charges. This trait contributes to its ability to buffer a solution without messing up cellular processes. HEPES stays solid up past 200°C and dissolves at 50 grams per liter in water, helping deliver solutions that keep steady around pH 7.2 to 7.6. A lot of researchers prefer it over Tris buffers, partly because its buffering doesn’t shift with temperature or concentration, which becomes especially important in sensitive cell cultures.
On shelves, a HEPES bottle shows its purity, batch number, and assay values, along with the chemical’s formula and recommended storage conditions—normally cool and dry. Purity above 99% matters for work in biotechnology, where even minor traces of contaminants can throw off a whole month’s worth of data. Real labs look for detailed certificates of analysis since these numbers determine if the batch works for molecular biology, tissue culture, or other high-stakes tests. Labeling mandates often call out allergens, storage temperature, and shelf life. I learned to check for heavy metal traces; just 0.1 ppm iron ruins certain enzyme assays.
Typical HEPES manufacture starts with the reaction between piperazine and ethylene oxide, followed by sulfonation with ethanesulfonic acid. Chemists refine the product further using repeated crystallizations and filtration steps. For a long stretch, only a handful of manufacturers controlled the process closely enough to deliver research-grade HEPES, but now the field looks a bit broader thanks to better quality control in emerging labs. Making a typical 1M HEPES buffer for biology uses careful weighing, slow addition of sodium hydroxide to reach the target pH, and filtration to achieve sterility. Homemade solutions should never sit out too long since degradation creeps in when exposed to UV light or high heat.
HEPES holds up against a lot of chemical abuse. It resists oxidation and avoids breaking down during many reactions, keeping cultures safe and happy. For special experiments, scientists have attached fluorescent labels to track molecules, or added other chemical “handles” for making new probes. HEPES can undergo esterification or can react with certain crosslinkers for polymer applications, especially where cell encapsulation demands something both sturdy and biocompatible. In my hands, HEPES refuses to meddle with enzyme substrates, making it a no-drama sidekick for protein work.
People often just call it HEPES, but check safety sheets and catalogs for names like 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid or N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid). Some brands list it under codes or numbers, but the chemical structure gives it away every time. Industry insiders might even refer to it by CAS number 7365-45-9, especially during procurement or regulatory paperwork. In supply chains, short-hand names help minimize order errors, which can get expensive or dangerous if the buffer’s purity strays from standard.
Most people won’t see HEPES as especially hazardous, though dust in the air produces mild throat or eye irritation. Lab rules require gloves and eye protection even if the chemical isn’t known for wild toxicity. HEPES powder creates slick surfaces, so spills on the floor mean accidents. We always wash up after using any buffer, HEPES included, because long-term exposure to fine powders never brings health benefits. Regulations in Europe and North America ask suppliers to register safety data sheets and keep detailed transport records, ready for inspection.
Uses for HEPES feel endless at times. The buffer stabilizes the pH during mammalian cell growth, covers DNA and RNA work, and crops up in electrophysiology, fluorescence imaging, and protein purification. Keep it handy for any application where cell viability sits at the center, especially for mammalian and hybridoma cell lines. Blood plasma and some clinical assays depend on consistent pH control, and HEPES steps in without altering sodium or potassium levels. From stem cell work to immunology, I see HEPES underline trustworthy results across dozens of projects.
HEPES sits at the core of countless studies aiming to refine cell culture growth and stem cell engineering. Pharmaceutical companies depend on this buffer to scale up biologics, since even small shifts in pH during production lead to failed batches that cost millions. Biomedical engineers seeking new drug delivery systems often rely on HEPES’ predictable performance to maintain solution pH under real-world conditions. Some labs have pushed into developing HEPES derivatives tuned for extreme environments—high heat, oxidative stress, or low ionic strength—fueling the next jump in cell therapy and diagnostics.
Researchers found that HEPES causes little acute toxicity in standard exposures, but concerns grow at high concentrations or after repeated, daily use. Some studies in animal models show altered kidney and liver functions at doses far above what most experiments use. More troubling, breakdown products formed under strong UV light or in high heat can produce small amounts of toxic sulfonates. We keep concentrations below 20mM for sensitive work, and always flush solutions thoroughly from cultures before in vivo studies to avoid complications. Keeping up with new regulatory data protects workers, but so does careful disposal—nothing good comes from dumping extra buffer down the drain.
The next wave of HEPES use looks set to move beyond its roots as a simple buffer. Work on functionalized versions aims to build sensing applications, stable coatings for medical implants, and new delivery methods for fragile therapeutics. Environmental safety groups look at long-term persistence and breakdown, suggesting new biodegradable HEPES analogs might come from green chemistry labs. Digital manufacturing, including automated tissue engineering and high-throughput screening, will likely need even tighter specs for purity, driving larger and more traceable supply chains. As research shifts to more complex cell models and regenerative medicine, I expect HEPES-based systems to adapt, solving hurdles from solubility to real-time imaging.
Labs keep a small group of chemicals on hand that quietly make or break experiments. 4-(2-Hydroxyethyl)Piperazin-1-Ylethanesulphonic acid, known by most as HEPES, places high on that list. Many scientists remember the challenges of keeping cells alive or enzymes working just right, running into the smallest shift in pH that could stop months of work in its tracks. HEPES enters these stories as a prime buffer, trusted in thousands of research labs.
HEPES was developed in the 1960s during a big push to find better buffers for biological experiments. Unlike simple salts, HEPES keeps pH steady even if temperature changes or if the system is exposed to air. I’ve seen many students try to use phosphate buffers or Tris, often battling fluctuating readings or frustration when cells stop behaving. HEPES doesn’t react with calcium or magnesium like phosphate does, and it won’t swing pH erratically if carbon dioxide drifts through a culture dish. This means researchers can grow delicate human cells or run enzyme assays without the headaches that come from more basic buffer choices.
Cell culture specialists choose HEPES for demanding jobs. Animal cells, for instance, do not forgive sloppy pH. HEPES works well in media for growing these cells because it supports stable growth even outside CO₂ incubators or in tissue engineering studies. Scientists working on new medicines trust that their data stays consistent. Without a reliable buffer, false results pop up and breakthroughs grind to a halt.
Biochemistry classrooms often use HEPES in experiments that need exact pH control. For example, in protein purification steps or measuring enzyme action, HEPES does its job quietly, letting researchers focus on discovery. Early biotechnology companies used HEPES in developing monoclonal antibodies and vaccine studies. That reputation stuck. There’s comfort in opening a bottle of clear HEPES solution, knowing hundreds of peer-reviewed articles proved it works.
Many folks in research grew up hearing that some lab chemicals pollute or are hard to dispose of safely. HEPES isn’t perfect—it doesn’t biodegrade easily and waste handling requires care. Some institutions explore greener alternatives for large-scale production, hoping to keep the benefits without overloading waste facilities. Hospital and biotech lab directors keep a close eye on how much HEPES they use and encourage thoughtful disposal.
The push for safer, more sustainable lab chemicals keeps growing. Some startups and academic labs experiment with different zwitterionic buffers, but HEPES maintains loyal followers thanks to simple preparation, stability, and compatibility with living systems. As more researchers share their experiences, the whole community stands to benefit from open discussions about pros, cons, and best practices for using chemical buffers in science and medicine.
People often look at a package, glance at the branding or the expiry date, but skip the small print about how to store it. That small section shapes the quality, safety, and shelf-life of groceries, medicines, even household chemicals. My own kitchen has taught me this lesson the hard way. Leaving a jar of salsa next to the stove once left me scraping crusty, moldy goop off my nachos. I didn’t think twice about the temperature it needed. Since then, a bit of attention to storage instructions changed how long my food and supplies last.
Warmth and cold aren’t only about personal comfort—they drive chemical changes. For foods, room temperature doesn’t suit every product. Milk out on the table turns sour in hours. Chocolates left in a hot car become a smear of cocoa butter and disappointment. Pharmaceuticals suffer even more from unstable environments. According to the World Health Organization, many medicines lose their potency if they’re stored above 25°C. Vaccines spoil outright if stuck in a regular fridge instead of special medical ones. Keeping things within recommended temperature ranges prevents waste and danger.
Damp air breeds mold, clumps powders, and tarnishes tablets. I’ve seen bags of sugar transform into sticky bricks after a muggy week. Fungi start growing in pantry bread. In pharmacies, tablets exposed to humidity break down faster, reducing their effect. Producers and sellers rely on dry, cool spaces, not just because it’s tradition—they’re fighting off spoilage with every decision. U.S. Food and Drug Administration guidance suggests keeping most medications in low-moisture areas, tightly sealed to limit air exposure.
Direct sunlight isn’t a friend to much beyond solar panels and succulents. Ultraviolet rays break down certain vitamins and drugs. That’s why so many products—olive oil, pain relievers, even syrups—sit in dark or opaque bottles. I learned quickly not to keep vitamins by my window; their color faded, and I couldn’t trust they still worked as promised. Proper storage isn’t about aesthetics; it's part of keeping products safe and effective.
Most people don’t have labs or high-tech climate controls at home. Still, a cool cabinet away from appliances protects foods and medications better than a sunny ledge or a cluttered counter. Using airtight containers plays a role too. For households without air conditioning, storing food and medicine in the lowest part of the house helps, as basements and lower cabinets stay cooler.
Stores and warehouses, of course, take things to another level. They track shelf temperatures, rotate stock to avoid forgotten items, and monitor for humidity. Some products require refrigeration from manufacturer to market, tracked by temperature loggers. Supermarkets and pharmacies follow tight guidelines and train staff to spot potential problems.
Many people wait for an obvious warning—bad smells, off colors, crumbling textures—before they suspect storage problems. With sensitive products, those signs show up after they’ve lost quality. Reading storage labels leads to fewer ruined dinners and keeps medications working as doctors intend. Paying attention to how and where we store things extends their usefulness and keeps us safe.
Many people in the lab want miracles from new compounds. I know the excitement of discovering a fresh reagent or building block and imagining breakthroughs in biology. There’s real hope, too, because a single molecule can open doors for therapies or diagnostics the field needs. Before dreams take over, one question stands between idea and result: will this compound behave in a living system?
Cells don’t tolerate everything—they’re not just tiny test tubes. My days mixing chemicals into media have taught me that even subtle changes in pH, solvents, or impurities shift growth and response in cell lines. A compound’s published data seldom show the full story. Beyond basic structure, factors like salt form, solubility, and carrier solvent set the tone for successful experiments. A lot of commercial chemicals come with invisible hitchhikers—trace metals, leftover synthesis byproducts, or stabilizers—which poorly documented suppliers might never mention.
Cytotoxicity is the obvious risk but not the only one. Some compounds interrupt cellular machinery in ways that might not immediately kill cells but quietly alter their behavior. Take simple DMSO, for example: above half a percent, it slows cell division and changes gene expression. Reports show that one in five “hit” compounds from small screens come up toxic or unstable under culture conditions. Researchers sometimes miss this, chasing false positives until repeated failures force a rethink. I’ve seen promising results fall apart because a molecule oxidizes in air or light, or interacts with plastic ware in subtle ways.
Evidence matters. Google’s E-E-A-T principles encourage us not to take vendor claims at face value. I look for peer-reviewed articles testing the compound in real biological systems. Even better, I value papers using the same cell line and solvent. If references are thin, a quick check in resources like R&D Systems or the ATCC compound database provides clues about reported use. Reviews or meta-analyses showing patterns for similar structures can be useful, too. It’s never enough to simply trust a chemical’s purity or label—batch-to-batch variation sometimes affects outcomes, so I keep test notes and always run basic quality controls.
Considering compatibility means planning controls and pilot experiments. I always leave room in my plate or flask for a solvent control and for cells without the test compound. Learning from failed experiments, I now run small-scale dose-response tests before scaling up. Buying only from reputable suppliers saves headaches, since reliable documentation and certificates of analysis make contamination less likely. For difficult compounds, I call technical support or reach out to colleagues with specific experience.
Cell culture media have quirks, and biological assays can react unpredictably to experimental tweaks. Phone calls with collaborators and a willingness to share “what went wrong” stories help others avoid the same traps. My experience shows that even ordinary substances can disrupt cell culture if handled poorly. Sometimes, the safest compounds in chemistry turn into biological troublemakers.
Before claiming success, I document every step—concentration, solvent, incubation time. If an unexpected effect turns up, repeating the experiment and swapping out the reagent often clarifies whether it’s a fluke or a true biological signal. The scientific community benefits when researchers share failed attempts as well as victories. Colleagues trust conclusions more when the story includes both the interesting outcome and the messier, negative results.
Responsible use and honest reporting build up knowledge, saving everyone time and resources. In my experience, taking extra steps to confirm compound compatibility pays off, turning hopeful experiments into real progress.
4-(2-Hydroxyethyl)piperazin-1-ylethanesulphonic acid, better known as HEPES, stands as a backbone in labs where pH stability matters. Anyone who has spent hours fine-tuning cell culture media or working with protein samples knows that temperature shifts or carbon dioxide exposure can mess up a good experiment in a heartbeat. Here, the choice of buffer sets the groundwork for meaningful results. HEPES earned a spot in the toolbox because it keeps pH steady between 6.8 and 8.2—right where many biological systems function best. From my own bench work, I’ve seen how a poor buffer lets pH drift and ruins a good set of cell responses or enzyme activity readings. Reliable HEPES makes experiments less stressful.
It’s common to start with HEPES in powder form. Lab routines kick off with weighing out the desired amount—precision balances matter, especially at high concentrations like 1 M. I always check the bottle for clumps; moisture can sneak in and throw off the weight. After the powder hits the mixing beaker, adding distilled or deionized water comes next. It pays to use freshly opened bottles and high-purity water, as contaminants will muddy the pH and potentially the experiment’s outcome. I’ve seen first-year students dump buffer straight into tap water, only to end up with unpredictable pH and wasted time.
HEPES dissolves well, though larger quantities sometimes resist full mixing. Magnetic stir bars or gentle swirling work much better than shaking the bottle and risking spills. Heating isn’t really necessary here. Once dissolved, the real skill comes into play: pH adjustment. Adding sodium hydroxide (NaOH) or hydrochloric acid (HCl) bit by bit while carefully watching the pH meter makes or breaks buffer quality. Overshooting the mark wastes buffer and interrupts workflow. Trained eyes recognize when it’s time to slow down, adding a few drops at a time as the pH creeps up to the target. I always calibrate my pH meter before starting, as old probes give false readings, which wastes valuable reagents.
HEPES solutions don’t play well with light; they eventually turn yellow and degrade. I always reach for amber bottles and store buffers in cool, dark places. It saves the trouble of remaking solutions on short notice and cuts costs. Some ignore expiration dates, but from hard lessons, I’ve learned degraded buffers misbehave, especially with sensitive cell lines or enzymes. Filtration through a 0.22 μm filter removes microbes and particles, much better than relying on old-school autoclaving, which damages the buffer’s structure.
Sometimes people just rely on recipes, skipping checks that protect experiments from disaster. Water quality and pH meter calibration come first every time. For labs in regions with hard water, purification systems deserve investment. Labeling bottles with preparation date, concentration, and who made it cuts confusion during busy days. If a protocol fails, the first step should always include confirming buffer integrity—much like double-checking ingredients before baking. It saves energy, money, and professional reputation in the long run.
A good buffer is only as trustworthy as the habits behind its preparation. Consistency, clean technique, and proper storage turn a chemical name like HEPES from a mere supply order into the foundation for reproducible, publishable science. Those habits take root through shared stories, lived lessons, and a willingness to do it the right way, every time.
Anyone who’s mixed up a powdered drink, tinkered with a cleaning solution, or adjusted the water in an aquarium has brushed up against the concept of pH, whether they realized it or not. Measuring how acidic or alkaline something is might seem like science class trivia, but staying in a safe pH range has a big impact on everything from safety to product effectiveness.
The typical product in this conversation — based on a wide swath of household and industrial goods — usually lands with its solution in a pH window of about 6 to 8. Anything much lower starts slipping into the acidic arena, which can corrode surfaces or sting skin. Moving higher takes you into alkaline territory, not great for things like sensitive electronics, certain dyes, or people’s hands. In my years working with general cleaning agents and some modest dabbling in hydroponics, products aimed for this neutral-ish range show up every single time. A handful of pool cleaners and bathroom scrubs get more aggressive, but managers and parents alike breathe easier inside this band.
Science backs it up — most dissolved substances found at home or work don’t push their pH too far from neutral unless strong acids or bases get involved. Many daily products rely on moderate pH so that pipes, floors, or even our stomachs don't get an unpleasant surprise. Take soap, for instance: it’s engineered close to neutral to avoid drying out skin. Cleaners balanced in this zone do not eat away common building materials. Even water straight from the tap hovers around 7 in most places because distribution systems and regulators learned that going outside these bounds shortens plumbing lifespans and opens up a world of headaches.
The U.S. Environmental Protection Agency recommends drinking water fall between 6.5 and 8.5. Most food-safe preservatives and food-grade mixes seem designed with those same boundaries in mind. I’ve consulted Material Safety Data Sheets for plenty of chemicals and the listed pH often sits squarely between those numbers, unless the product is designed specifically to be caustic or highly acidic for a clear reason. Home pH test strips purchased at any garden supply, pool supply, or pharmacy store tend to measure precisely this range because that’s where most people actually need to pay attention.
Anybody mixing up a solution and spotting pH outside the safe band ought to double-check directions, their instruments, or the product’s integrity. Contamination, expired ingredients, or plain old measurement errors all cause weird readings. Adding more water can pull an overly acidic or basic mixture back to safer territory, but sometimes fresh ingredients work best. If a consistent off-kilter pH shows up, contacting customer service or jumping onto the manufacturer's site proves useful. Plenty of companies post troubleshooting guides for just these situations. Staying up to date with product recalls or safety alerts won’t hurt, either.
Plenty of manufacturers tinker with formulas to keep solutions inside this Goldilocks pH zone, partly to meet stricter regulations and partly to address user feedback. Newer "green" cleaning products, for instance, advertise neutral pH as a badge of consumer safety. On the food side, restaurant inspectors use simple color-coded pH strips as a frontline defense against accidental spoilage or dangerous mixing mistakes. As testing grows ever simpler and more affordable, expecting transparent reporting on pH shouldn’t feel like too much to ask.
Bottom line: Paying attention to pH isn’t just for scientists—it's a practical choice anyone can make for health, safety, and better results at home or work.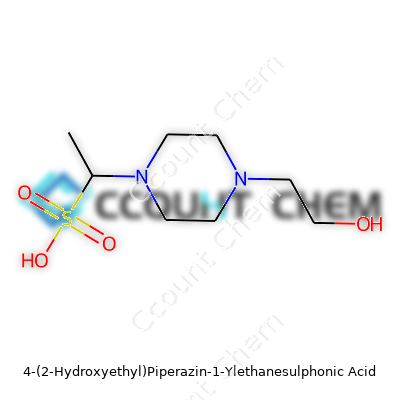
| Names | |
| Preferred IUPAC name | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| Other names |
HEPES N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid N-(2-Hydroxyethyl)piperazineethanesulfonic acid Hepes free acid |
| Pronunciation | /ˈhajdroʊˌɛθɪl.paɪˈpɛrəˌzin.wʌn.ɪˌleθ.eɪn.sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 7365-45-9 |
| 3D model (JSmol) | `load =1zuv` |
| Beilstein Reference | 1718734 |
| ChEBI | CHEBI:39097 |
| ChEMBL | CHEMBL1138 |
| ChemSpider | 4701 |
| DrugBank | DB03742 |
| ECHA InfoCard | 03b51eaf-ef1a-4b7c-bf43-153290c0d62d |
| EC Number | EC 219-022-2 |
| Gmelin Reference | 1842224 |
| KEGG | C01841 |
| MeSH | D010572 |
| PubChem CID | 2389 |
| RTECS number | TD1275000 |
| UNII | J3R93D7K2A |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID8014269 |
| Properties | |
| Chemical formula | C8H18N2O4S |
| Molar mass | 238.301 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.11 g/cm³ |
| Solubility in water | soluble |
| log P | -3.5 |
| Vapor pressure | <0.0000001 mmHg (25 °C) |
| Acidity (pKa) | pKa = 7.5 |
| Basicity (pKb) | 9.78 |
| Magnetic susceptibility (χ) | -47.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.510 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.28 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1407.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2203.8 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 Oral Rat > 10,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): > 5,000 mg/kg (rat, oral) |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 0.1-1.0 M |
| Related compounds | |
| Related compounds |
PIPES HEPES MES TES ACES BES CHES MOPS TAPS |