Laboratories once relied on simple buffers like phosphate or bicarbonate to manage pH in biochemical work. Problems cropped up fast. Old buffers didn’t always stay stable when the temperature went up, or enzymes started doing their work. Someone who spent time in the lab in the 1970s would remember researchers chasing after solutions that held steady without introducing new variables. Norman Good and his team in the mid-twentieth century went after these problems with a list of "Good’s buffers," focusing on compounds that wouldn’t pull calcium out of solutions or get in the way of cell growth. PIPES, HEPES, and MES came out of this period. Before long, 4-(2-hydroxyethyl)-1-piperazinepropanesulphonic acid—better known as EPPS—joined this family, brought to life by minds focused on smooth biological experimentation. Decades went by, but the value of this class of buffers stuck around. EPPS went from a specialty product to a staple on the shelf for anyone who needed protein or cell biology to work as planned.
Most researchers know 4-(2-hydroxyethyl)-1-piperazinepropanesulphonic acid by its shorter name, EPPS. EPPS belongs to a class of zwitterionic buffers, chemical compounds carrying a positive and a negative charge in the same molecule. Sitting in a white crystalline solid form, EPPS dissolves quickly into water, and doesn’t gum up with clumps like some other powder-based buffers. The main job for EPPS is to keep solutions stable between pH 7.0 and 8.5, a sweet spot for lots of enzyme work or culturing mammalian cells. Blended into life science workflows, EPPS rarely brings along unwanted chemical baggage—no toxic metals, no weird by-products, no pH drift that throws off results.
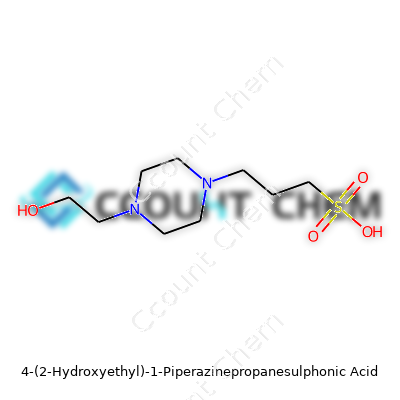
EPPS stands out by carrying both an amine group and a sulfonic acid moiety. Chemists put it in the camp of piperazine derivatives. The molecular formula is C9H20N2O4S, with a molar mass of 252.33 g/mol. Pour it onto a dish and you’ll see a white, odorless solid, quick to melt into clear solution. Water loves EPPS—solubility is high, so you can make concentrated stocks and not worry about undissolved grit. This buffer won’t boil away or break down at room temperature. The pKa sits at approximately 8.0 at 25°C—a practical window for protein biochemistry, DNA work, or electrophoresis. Once mixed, EPPS won’t mess with most metal ions or leach out trace contaminants, sidestepping hassles common with less specialized buffers.
Chemical supply companies ship EPPS labeled with its CAS Registry Number, 16052-06-5, and batch-specific purity data, most often topping 99%. Material Safety Data Sheets include detailed composition, storage instructions, and hazard symbols for transparency. Shelf-life guidance tells labs to dry-store EPPS at controlled temperatures, away from light and air. Certificates of Analysis often accompany every batch. Container labels come with pH range, recommended concentration, and chemical-grade (such as molecular biology or analytical). Practical folks read these specs closely to avoid mix-ups, especially when working at scale or troubleshooting a sticky assay.
Synthesizing EPPS involves reacting 1-piperazinepropanesulfonic acid with ethylene oxide under carefully controlled conditions. Producers favor water-based, low-toxicity procedures—traditional organic solvents do not play a part in responsible manufacturing. Steps include pH control during alkylation, careful temperature monitoring, and end-stage purifications to strip away unreacted side-products. Experience in small-scale organic chemistry translates well; any scale-up demands attention to reactor materials, agitation speed, and temperature checks. Quality depends on rigorous purification, not shortcuts, because traces of by-products or metal contaminants can throw off sensitive biological reactions down the road.
Scientists rarely reach for EPPS to carry out synthetic work—this buffer’s decked out to stay inert, holding a steady pH as enzymes or cell lines do the heavy lifting. Even so, the hydroxyl and piperazine groups offer a few handles for derivatization. Some researchers attach labels or sensors to EPPS to track transport in cells. Strong oxidizers or acids do break EPPS down, so storage and usage need a bit of respect. EPPS won’t grab onto most metal ions, thanks to its design, so you can trust it not to bind away cofactors or misbehave in buffer solutions that call for transition metals, important for enzymatic reactions.
Beyond EPPS, you may spot 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid in catalogues, or the mouthful HEPPES. Some vendors label their products "Good’s buffer EPPS" to put the legacy front and center. Specialty grades, like "Ultra Pure EPPS" or "Biotech Grade EPPS," often flag intended use or purity. Batch numbers, packaging size, and application notes round out supplier listings. Still, "EPPS" remains the shorthand that nearly everyone understands at a glance.
Working with EPPS means paying attention to dust formation and direct skin or eye contact. Users often wear gloves and goggles to dodge respiratory or mucous membrane irritation, especially in dry powder form. This compound stays out of the list of acute environmental threats, and disposal through diluted aqueous waste fits most guidelines, but local regulations must always get checked to handle larger volumes. Each lab keeps an eye on storage—sealed containers, cool shelves, and avoidance of strong oxidizers go a long way. Emergency eyewash stations and spill kits belong in the same room as any buffer, not just for EPPS, but for any solid handled in bulk. Training and transparent labeling matter for new staff, since careless handling could expose folks to minor irritation.
EPPS brings value to cell physiology, molecular biology, protein crystallization, and electrophoresis. Its stable pH window, clean behavior in the presence of magnesium or calcium, and easy mixing translate to more reproducible, sharper data. For work on sensitive proteins or cell cultures, researchers count on EPPS to avoid shifts in osmolarity or toxic ion build-up. DNA hybridization, PCR, and a wide range of enzyme assays run smoother with dependable buffering. In protein crystallization, EPPS lets users fine-tune pH without worrying about precipitation or salt interference. Academic labs and industrial biotech centers both count on this compound in everyday protocols.
Manufacturers and public sector labs continue looking for ways to boost purity, lower cost, and improve the environmental footprint of EPPS production. As molecular diagnostics and therapeutic protein manufacturing expand, demand for consistent, contaminant-free buffer systems climbs. Researchers tinker with formulation tweaks, lyophilized buffer packs, and single-use containers to trim contamination risk. EPPS shows up in patent applications for new immobilized enzyme platforms and diagnostic microfluidics. Academic chemists have explored structure-activity relationships to build more robust, cost-effective alternatives, but EPPS still holds firm ground as a proven performer.
Safety testing circles back to the classic values of "Good’s buffers," looking for low toxicity and lack of bioaccumulation. Large-scale studies and internal industry trials confirm EPPS does not break down into carcinogens or persistent pollutants. In vitro assays show mammalian cells tolerate typical buffer concentrations (10–100 mM) without growth inhibition or unexpected apoptosis. Still, a handful of published papers describe osmotic or metabolic stress if researchers crank up buffer concentrations too high. Lab workers who have accidentally tasted or inhaled buffer dust report mild, temporary irritation. Environmental persistence studies show quick breakdown in sewage treatment, without lasting effects.
Some of the next steps for EPPS and related buffers follow the spread of cell therapies, advanced pharmaceuticals, and precision diagnostics. Automation and high-throughput workflows drive demand for even tighter controls on purity and consistency. Companies now explore biodegradable packaging, greener synthesis, and better analytical methods for impurity profiles. Researchers will keep testing buffer capacity under ever-tougher conditions—temperature swings, enzymatic cocktails, or extreme scaling. Bioengineers hope to tune buffer properties further, perhaps through new chemical tweaks to support synthetic biology or integrative omics. The story of EPPS continues wherever science needs reproducible, reliable buffering in a world pushing for less waste and more precision.
Scientists in labs often depend on reliable foundations. In the world of biological research, that foundation for many experiments comes in the form of a buffer—the kind of chemical that keeps a solution's pH stable. One of the most dependable choices over the years has been a compound with the long name 4-(2-Hydroxyethyl)-1-piperazinepropanesulphonic acid. Researchers usually call it by its shorter name, HEPPS or EPPS.
Long days in a lab have shown me how temperamental biological systems can be. The tiniest shift in pH can send enzymes into chaos or wreck a protein’s structure. For a researcher, uncertainty can burn precious time and resources. Here’s where HEPPS steps in. This acid keeps the pH in a steady range, usually around pH 8. It gets used in many protein studies, enzyme experiments, and cell cultures. What makes it stand out is its low reactivity. HEPPS won’t interfere or react strangely when mixed with other chemicals. This gives scientists a calm, predictable environment to work with.
A few years back, I watched a colleague tweak cell growth conditions for weeks before he realized the buffer he picked was messing with his results. He switched to HEPPS, and the stability it offered took away a whole layer of guesswork. Today, labs use this buffer for growing bacteria to produce proteins, for running enzyme-linked immunosorbent assays (ELISA), and for purifying certain biological molecules. Doctors’ offices may benefit, too: kits designed to test blood or urine sometimes use HEPPS to keep things reliable from batch to batch.
Buffers like HEPPS support accuracy in disease detection. Doctors trust these tools to check for conditions such as diabetes or kidney problems. Mistakes here could steer treatment the wrong way. For people depending on those results, every layer of reliability counts. Publications and quality assurance reports back up HEPPS as a solid performer with low toxicity. When companies look to improve medical tests, choosing tried-and-true buffers helps keep patient care safe.
Reliable products rarely come cheap. HEPPS costs a bit more than basic options, but the stability pays off in the long run. Labs waste less time repeating failed experiments. There’s a trade-off, though. Manufacturing chemicals brings some environmental weight. The industry is moving slowly but surely toward greener chemistry. Some researchers are designing recycling systems for used buffer or hunting for more biodegradable alternatives without losing performance.
Most people outside the sciences never hear about these compounds. Thinking ahead, science education could improve by pulling back the curtain. Students need to understand not just the science but also the silent workhorses like HEPPS that make breakthroughs possible. Funders and lab managers can push for suppliers who value both performance and sustainability.
Buffers often don’t grab headlines, but 4-(2-Hydroxyethyl)-1-piperazinepropanesulphonic acid quietly powers progress across biology and medicine. Countless experiments—and the lives they touch—rely on this consistent, unassuming chemical. Smart choices at the chemical bench echo much further than the lab walls.
HEPPS, also called EPPS or 4-(2-Hydroxyethyl)-1-Piperazinepropanesulphonic Acid, isn’t some mystery ingredient found only in remote laboratories. This compound lands on the shelves of many research settings, especially for biochemistry and cell biology work. People rely on its stable buffering performance in key lab environments. It’s not the buffer everyone chooses out of habit—researchers reach for it because it actually gets results, keeping pH levels on track during sensitive experiments.
If you’ve spent any time in a wet lab, you know the drill: weighing out chemicals with precision keeps everything running smoothly. HEPPS is no exception. Its molecular weight—238.3 grams per mole—might look like just another number, but that number underpins accurate solution prep. Make a mistake or round up on the calculation, and your experiment could yield a mess of confusing results, wasted time, and more frustration than answers. It’s surprising how much relying on a correct molecular weight cuts down on headaches later. Most buffers get mixed from scratch, so there’s no room for errors hiding in the background.
HEPPS sits in a group with other “Good’s buffers”—compounds recognized for their low reactivity, predictable performance, and low absorption in critical measurement ranges. Folks working on protein purification, enzymatic assays, or cell culture like it because it resists the urge to react with metals or enzymes. Instead, it quietly does its job, stabilizing the experiment without getting in the way. This kind of reliability builds trust. Research publications, lab protocols, and suppliers all cite the same molecular weight, reinforcing that 238.3 g/mol value as the standard. Nobody wants to hunt for the right numbers every time a colleague mixes a solution.
It’s not enough for manufacturers or suppliers to slap a number on their packaging. Researchers count on traceable, consistent product information. Mistakes in chemical catalogs or discrepancies between suppliers can ruin batches of samples, waste precious reagents, or even endanger experiments involving living cells. Energy spent double-checking or re-calculating doesn’t feel productive. What matters is using data tied to trusted databases and established chemistry references. Reproducibility in science leans on everyone in the process, from companies handling manufacturing all the way to the student prepping their first buffer.
Setting clear, transparent documentation for each compound supports the whole scientific ecosystem. Teachers can help new lab members understand not just how much to weigh, but why precision matters. Labs cut down on errors by sticking with reliable sourcing, checking reagent purity, and documenting every prep. Industry organizations and standards bodies help create the shared language that keeps researchers on target, whether in academic labs or industry R&D. Leveraging trusted resources builds better science, with dependable results at every stage from basic prep to published outcomes.
Quality chemicals and reliable documentation give research scalability and reproducibility. In my own lab experience, nothing feels better than seeing clean results after a careful buffer prep using the right numbers. Small steps—like double-checking the molecular weight on the label—make a big difference for the whole scientific community. HEPPS and its specific weight play a part in the process that shapes breakthroughs, not just for one lab, but for the wider world relying on honest, transparent science.
4-(2-Hydroxyethyl)-1-Piperazinepropanesulphonic Acid rolls off the tongue about as well as a highway covered in black ice, so most labs just say HEPPS. This buffer has carved its niche in the world of biochemistry. Each buffer aims for a sweet spot, and HEPPS calls the pH range of 7.3 to 8.7 home. I started out pipetting all the usual suspects, but the first time I reached for HEPPS, it was clear this wasn’t just another bottle on the shelf.
Most folks walk through life without thinking twice about pH, but in a research lab, it can make or break an experiment. Enzymes, proteins, and even cells push back when the pH isn’t just right. My early days working with protein assays drilled that lesson home. One test worked perfectly at pH 7.5 but fell apart as soon as the buffer tipped closer to 8.5. HEPPS shines for those working between 7.3 and 8.7. Many proteins seem happiest right there, and that saves a lot of troubleshooting headaches.
Buffers range from simple to complex, but each one centers around its pKa—the tipping point where half the molecules have released a proton. For HEPPS, the pKa sits near 8.0 at 25°C. That marks its playground. If you mix up a buffer solution using HEPPS, you can count on it to hold its ground across that narrow but crucial band. For labs running DNA or protein work in slightly alkaline conditions, HEPPS beats many options. Tris buffers often get plenty of attention, but their pH can shift if the temperature or ionic strength changes too much. HEPPS doesn’t waver nearly as much in similar setups. Researchers trust that reliability.
Studies in protein crystallization, enzyme kinetics, and even cell culture work often list HEPPS among the chosen buffers for alkaline settings. Research published in the Journal of Biological Chemistry cites its stability under heat and salt stress. One project I joined tracked activity of bacterial enzymes, and we found that cells performed their best in HEPPS buffer, as long as the pH stayed tight around 7.5 to 8.5. Sudden changes slowed things down, but consistent pH let us trust our data.
Getting the right buffer can feel like chasing your own tail. Not every lab keeps HEPPS on hand since it’s pricier than simple options like phosphate. Some colleagues make do with what’s available, sometimes stretching a buffer past its ideal pH. That tends to bring on trouble later. If budgets allowed for a few more specialty buffers, research would dodge plenty of repeat experiments and wasted days. Using the right buffer for the pH range cuts down on sample variability and boosts confidence in the outcome.
Working with biological systems means every detail counts, from the pipette tips to the buffer inside that clear flask. Knowledge isn’t just memorizing facts; it’s remembering which buffer changed the game for a project. HEPPS holds a stable line around pH 7.3 to 8.7 and that can kick open doors otherwise left shut by unreliable pH. Picking a buffer isn’t glamorous, but it can turn a tedious experiment into a discovery. For many labs chasing answers in slightly alkaline waters, HEPPS keeps the current steady.
The world of chemistry carries tongue-twisters like 4-(2-Hydroxyethyl)-1-Piperazinepropanesulphonic Acid, known among chemists as HEPPS. This compound usually pops up in labs, especially where researchers need to keep biological samples stable. The structure — a piperazine ring with hydroxyethyl and propanesulphonic acid side chains — isn’t just for show. Each part shapes how the acid behaves in different environments.
Everyday life feels distant from bench-top science, but the principles run parallel. Solubility comes down to how the pieces of a molecule interact with water’s charged sides. Unlike table salt that dissolves easily, some compounds resist the urge to mingle. But HEPPS brings a sulfonic acid group and a hydroxyethyl handle, both eager to form bonds with water. I remember pouring HEPPS powder into beakers to prepare buffer solutions; it always vanished into the mix with gentle stirring, giving no excuse for clumps or undissolved grit. Years of lab experience back up published reports: HEPPS demonstrates strong solubility in water under normal lab conditions.
Research literature, including standard biochemical reference handbooks, lists solubility values comfortably above 100 grams per liter at room temperature. This isn’t trivial. Many biological reactions hinge on a buffer’s ability to dissolve completely, without cloudiness or leftovers. Reliable solubility means researchers don’t need high temperatures or fancy solvents to coax the powder into solution. The molecular design with hydrophilic (water-loving) groups lends itself to straightforward, hassle-free preparation.
Consistency and reproducibility drive every good experiment. Unpredictable buffers sow confusion, eat up time, and waste resources. Easily dissolved HEPPS streamlines things — no gritty textures, no repeated filtering. During crowded, fast-paced days in the lab, being able to trust your buffer mix lets you focus on the bigger questions. Anyone working at the bench understands the frustration of waiting on a stubborn chemical to finally disappear, watching the clock tick toward a missed appointment or lost daylight.
Beyond biology, solutions that behave predictably matter for companies formulating products like pharmaceuticals, cosmetics, and cleaning agents. The easy mixability of HEPPS gives it an advantage. Manufacturers find fewer headaches in scaling up preparations, especially for products regulated on stability and quality.
Storing or mixing HEPPS with other chemicals isn’t a completely risk-free affair. Overly acidic or basic solutions can shift the chemical landscape. Occasionally, impurities or excessive concentrations may slow down the process or shift the pH unexpectedly. Good practice means checking each new batch for consistency, running quick tests, or tracking lot numbers — a practice recommended by major chemical suppliers. To keep things straightforward: stick to distilled water, stick to reasonable concentrations, and solutions behave as expected.
Researchers who encounter issues with incomplete dissolution should double-check the water quality, avoid overloading the solution, and stir with patience. Lightweight heating (not boiling) can sometimes help, but most routine situations never need such tactics. Equipment cleanliness and accurate weighing matter, too. A digital stirring plate and clear glassware can prevent most of the little setbacks that add frustration to an otherwise simple task.
Plenty of labs depend on buffering agents like 4-(2-Hydroxyethyl)-1-Piperazinepropanesulphonic Acid, often simply called EPPS. It works wonders for keeping pH stable, especially around biological samples. Folks often reach for it in biochemistry, cell culture, and diagnostic kits. The science behind why EPPS does a good job is solid. Researchers count on this stuff to keep their experiments reproducible and their cells alive. But a reliable buffer still deserves respect in every step—storage, mixing, and disposal all ask for consistent safety standards.
EPPS, in pure form or concentrated solutions, can cause problems if someone gets sloppy. The main risks come from direct skin or eye contact. Labs sometimes forget gloves for quick jobs, then deal with stinging hands or worse after a spill. Breathing in powder during weighing or mixing isn’t wise either. It may not give off strong fumes, but dust exposure can irritate lungs, especially for folks with asthma.
MSDS sheets back this up—they show EPPS as an irritant. Manufacturers warn not to let it get on your skin or in your eyes, which is true for a huge number of lab chemicals. That’s not dramatic, but it matters because careless habits add up. Cutting corners with PPE or ignoring a splash may not hurt right away, but over years, that attitude changes how safe a lab feels. Once someone needs a trip to urgent care over a spilled buffer, the point gets driven home quickly.
People often forget about what happens after pouring something down the drain. EPPS doesn’t top the list of most toxic substances, and in small quantities, water treatment handles it just fine. Someone working through liters every month should pause before treating it like table salt. For bigger labs, local disposal rules often ask for neutralization or designated chemical waste collection. Even if there’s no obvious harm, best habits keep waste streams clean and help science. Labs that respect these rules show the next generation how chemical handling should look.
Gloves, goggles, and lab coats do more than make for a glossy safety poster. Wearing all three turns into a routine—for new students and old hands alike. I once watched a seasoned tech get careless, skipping gloves for a routine buffer prep. In under a minute, a light splash set off a chain of handwashing, paperwork, and, most of all, a day of irritation that hurt more than a simple reminder would have. Respect for protective gear starts with lead scientists modeling good habits.
Clean-up routines also matter. Even tidy labs can fall apart if everyone assumes the last person wiped the bench. Assigning clear roles for chemical clean-up, regularly checking the spill kit, and actually running through spill drills build a culture that values safety. I’ve seen students roll their eyes at drills, but a real spill wipes that smirk pretty quickly. These are the messy truths that don’t make brochure copy but mean a lot to lab workers’ well-being.
Clear labeling, keeping up-to-date data sheets within arm’s reach, and making disposal bins easy to access—these all go further than any warning label. People forget what chemicals can do if the reminders fade into the background. Small steps, done every day, cut down on emergencies and build up trust across the team.
| Names | |
| Preferred IUPAC name | 4-[2-hydroxyethyl(piperazin-1-yl)]butane-1-sulfonic acid |
| Other names |
HEPPS EPPS 3-[4-(2-Hydroxyethyl)piperazin-1-yl]propanesulfonic acid |
| Pronunciation | /ˈfɔːr tuː haɪˈdrɒksɪˌiːθɪl wʌn paɪˈpɛrəziːnˌprəʊˈpeɪnsʌlˌfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 103404-87-1 |
| Beilstein Reference | 4123460 |
| ChEBI | CHEBI:38743 |
| ChEMBL | CHEMBL1247 |
| ChemSpider | 82132 |
| DrugBank | DB06896 |
| ECHA InfoCard | 03aaf177-03ed-498b-9f6f-5fd9b6edfe99 |
| EC Number | EC 252-487-6 |
| Gmelin Reference | 906391 |
| KEGG | C05951 |
| MeSH | D017929 |
| PubChem CID | 178253 |
| RTECS number | TC3506000 |
| UNII | ZYE08Y5X2C |
| UN number | UN number: "Not regulated |
| CompTox Dashboard (EPA) | DTXSID6014561 |
| Properties | |
| Chemical formula | C9H20N2O4S |
| Molar mass | 309.40 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.109 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.5 |
| Vapor pressure | <0.01 mmHg (20 °C) |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 9.55 |
| Magnetic susceptibility (χ) | -5.54·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.510 |
| Dipole moment | 6.9461 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 368.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1125.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1764 kJ mol⁻¹ |
| Hazards | |
| Main hazards | No significant hazards. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H318 |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Flash point | >100°C (212°F) |
| Lethal dose or concentration | LD50 Oral Rat 6000 mg/kg |
| LD50 (median dose) | LD50 (median dose): > 10,000 mg/kg (Rat, oral) |
| NIOSH | GBG2300000 |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
HEPES EPPS MES CHES ACES TES Tricine MOPS |