In the years since organic chemists began searching for better buffers, the arrival of 3-Morpholino Propanesulfonic Acid (MOPS) marked a turning point. Early pioneers, like Norman Good in the 1960s, knew that many biological assays suffered from unreliable pH control. With too much background activity or unpredictable outcomes, researchers needed more precise tools for their work. MOPS did not just appear overnight — its emergence tracked the growing importance of keeping biological reactions in a stable, repeatable environment.
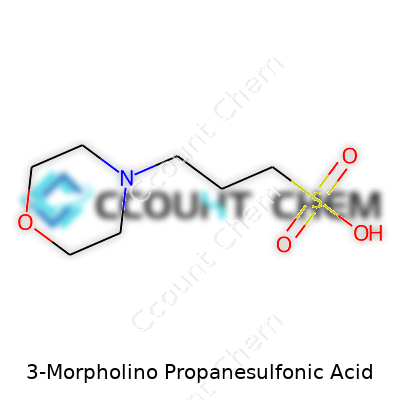
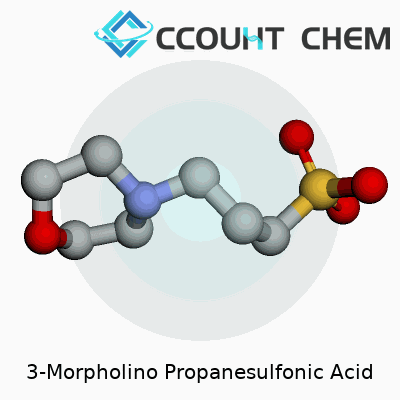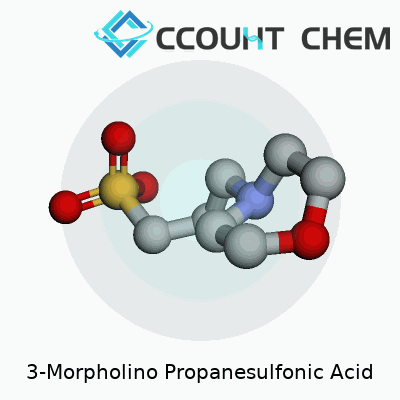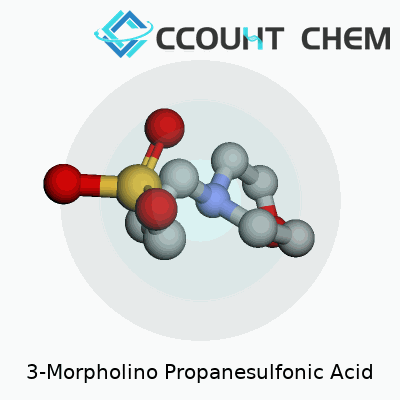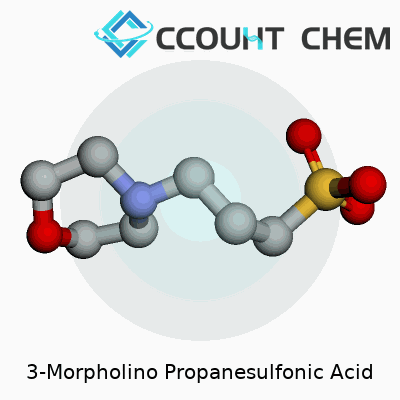
MOPS has an unassuming appearance: a white, crystalline powder with good solubility in water. Its structure combines a morpholine ring with a propanesulfonic acid group. This gives it a strong buffering action near pH 7, right in the physiological sweet spot where many proteins and enzymes function best. Chemically, it carries a molar mass of about 209 grams per mole and remains stable under most conditions a laboratory can throw at it. Compared to some older buffers like phosphate, MOPS doesn’t bind metals as much, so it avoids one stubborn issue in protein studies: keeping essential ions available without the buffer getting in the way.
Looking closer at specifications, MOPS usually comes at purity levels above 99%, with labeling to match international chemical standards such as CAS No. 1132-61-2. The acid dissolves quickly in water but not in typical organic solvents, so it stays in place during aqueous workings. Many bottles on the market list pKa values around 7.2 at 25°C, and each batch must meet heavy metal limits, usually below 0.001%. High-grade lots receive further checks for UV transparency, ensuring the buffer will not absorb light in key spectrophotometric assays.
Manufacturers often begin with morpholine and 1,3-propanesultone. Starting with morpholine, a common amine, chemists react it with an excess of 1,3-propanesultone, which opens up and attaches the sulfonic acid group to the terminal carbon. The result: a sulfonic acid with the desired morpholine ring intact, perfect for buffering. This method scales up easily, so plants can deliver MOPS on demand without a hitch.
Most users treat MOPS as a stable, inert buffer, but it does react under harsher conditions. Strong oxidizing agents or intense acid hydrolysis can knock out the morpholine group or sulfonic acid. In the lab, chemists sometimes chemically modify MOPS to tailor buffer capacity or change the solubility profile in custom applications. Still, for 99% of research, its straightforward behavior keeps things simple, as intended by its original inventors.
MOPS shows up under plenty of guises. Alongside "3-Morpholino Propanesulfonic Acid," people call it by its acronym, its salt forms like "MOPS-Na," or catalog numbers used by major suppliers. As research spilled across borders, synonyms such as "3-(N-Morpholino)propanesulfonic acid" began appearing, but they all point back to that same dependable molecule.
Labs demand high standards for anything that touches biological material. MOPS has, for decades, proven itself to be low in acute toxicity. Gloves and standard lab PPE keep routine handling worry-free, and spills pose only minor inconvenience, usually cleared with water and a wipe-down. Storage requires nothing special: a cool, dry spot away from strong acids and oxidizers. Regulators like OSHA have not found cause to add MOPS to restricted lists, and periodic safety reviews keep protocols current for workers both new and veteran.
MOPS buffers guide high-stakes experiments. Protein electrophoresis uses it to hold pH steady, so bands appear sharp and interpretable. Mammalian cell culture baths grow healthier thanks to its minimal metal binding and lack of biological interference. In environmental science, researchers test water samples with MOPS to avoid spurious results caused by less predictable buffers. Vaccine formulation, DNA sequencing prep, chromatography — these fields all rely on this dependable white powder. Having spent years on the research bench, I saw MOPS take up space on refrigerators, in autoclaves, and in every experimental design after other buffers had failed to keep an enzyme happy.
Researchers now push MOPS to new frontiers. The buffer’s stability draws attention for use in high-throughput diagnostics and advanced protein crystallography. Analytical chemists leverage its low UV absorbance to reach deeper detection limits. Meanwhile, synthetic biologists value MOPS for its integration into microfluidic devices, where tiny volumes and sensitive components cannot handle erratic pH shifts. Each year brings technical reports showing new tweaks, fresher applications, broader adoption in life science startups, and more detailed safety analytics.
Toxicologists put MOPS through tough scrutiny. Studies in cell cultures and rodent models show that, at practical concentrations, it does not disrupt growth or cause major cellular stress. Long-term waste management receives attention too. Since MOPS does not linger in waterways and does not bioaccumulate, it checks the right boxes for green chemistry. Still, careful disposal practices make sense because any chemical load in the environment merits respect, even for low-hazard materials.
Buffer chemistry is more than a solved problem — science will always want better precision, less interference, and easier integration with automation. As biochemical instrumentation advances, manufacturers work to make purer, more consistent MOPS, cut batch-to-batch variability, and offer pre-packed buffer kits to save time and reduce mistakes. Sustainable production and improved disposal remain crucial for a world more focused on environmental responsibility. As frontline researchers seek new ways to keep enzymes, cells, and biological products ticking, this buffer, supported by decades of reliability, will keep finding new jobs — both expected and surprising — across science and industry.
3-Morpholino Propanesulfonic Acid, known around the bench as MOPS, often pops up on lab supply orders for good reason. People in research and diagnostics know this compound has real value. MOPS stands out in the biology world because it helps keep pH steady during delicate experiments, especially for processes involving RNA. Ask anyone who has tried to separate RNA without a strong buffer and they’ll tell you—having the right chemical buffer makes the difference between messy data and clear results.
Labs that study gene expression or develop new diagnostic kits often reach for MOPS. A lot of lab routines look simple from the outside, but they hinge on steps that need steady conditions. Something as basic as measuring an enzyme’s activity can throw off results if the pH swings, which wastes time and resources. No one wants to repeat experiments or explain failed runs because of a weak buffer.
MOPS serves up dependable buffering, usually around pH 7, which suits many biological reactions. Scientists put their trust in it during electrophoresis, which separates molecules like DNA and RNA based on size. In fact, many commonly used buffers in agarose gel electrophoresis list MOPS as a main ingredient. The clean, neutral background MOPS helps maintain lets researchers spot even tiny fragments of RNA without distractions or breakdowns along the way.
Diagnostics depends on reliability. Lab professionals depend on MOPS for tests that look for infections or genetic changes. Take RT-PCR as an example. This method helped to detect COVID-19 during the pandemic. The results only work if the steps before the readout, such as preparing the sample and doing the reactions, go smoothly and without sudden pH changes. MOPS buffering helps keep those conditions constant.
Development teams in the pharmaceutical industry pay attention to ingredients like MOPS too. Stability in quality control assays gives companies evidence that new products work as intended. Flawed or inconsistent tests create delays. Regulatory agencies look for solid validation data, so a buffer that keeps things simple and robust matters.
There are always questions about the chemicals people use in the lab. MOPS does its job, but it’s important to handle it with care. Waste from testing labs accumulates quickly. Good practices—like tracking usage and spilling less—reduce environmental impact. Lab managers teach new staff about safe handling and disposal because it’s not just about safety today but about protecting the water supply downstream.
Sometimes people talk about switching to more eco-friendly buffer systems. So far, though, nothing beats the reliability and performance MOPS offers for certain methods. It helps that the science community shares best practices and new findings about greener options, keeping both reliability and sustainability on the radar.
MOPS won’t disappear from labs anytime soon. There’s still plenty of room to push for smarter use and safer alternatives in the future. Smaller batch sizes, automated mixing, and easier-to-handle packaging already help labs use only what they need, cutting down on waste. Suppliers can support this move by offering refills and supporting recycling initiatives.
Researchers and lab workers care about both science and safety. They keep an eye on what works in practice, not just in the manual. For now, MOPS remains an everyday hero of the bench, but it’s wise to keep asking questions and looking ahead to what could work even better—for results, for people, and for the planet.
3-Morpholino propanesulfonic acid, known to many lab workers as MOPS, carries the formula C7H15NO4S. People in biochemistry and molecular biology count on it to keep experiments running smoothly. This substance acts as a buffering agent. In my own time studying proteins, I reached for MOPS because its buffering range lands right where many key reactions happen; it reliably keeps pH levels steady, often from 7.0 to 7.4, keeping sensitive processes from getting derailed by shifts in acidity.
The string of elements—carbon, hydrogen, nitrogen, oxygen, sulfur—barely hints at the range of impact MOPS can have inside a test tube or fermenter. One little shift in pH can throw off months of work, so having a buffer like MOPS, which doesn’t react with most enzymes or metal ions, becomes crucial. Researchers trust it partly because its structure stops it from interfering with biochemical reactions, unlike some other buffers which bind calcium or disrupt cellular activity.
Students in college labs ask what separates MOPS from the common buffers like phosphate. With MOPS, bacteria and mammalian cells grow in a zone that supports both health and accurate data. Many DNA and RNA studies lean on it for this reason. Its chemical stability means the formula remains the same under harsh lab lights or after many cycles through freezing and thawing. I recall trying weaker buffers, and each time, the variability crept into the results until MOPS replaced them—a straight illustration of how one specific formula leads to more reliable science.
Trust builds up around chemicals that perform as promised. Researchers, educators, and companies rely on substances that support transparent, repeatable results. As lab work forms the foundation for medical discoveries or food safety, accuracy can’t slip. From an experience angle, people talk about the headaches of restarting research when a poorly chosen buffer lets pH swing out of control. Consistent results and clear guidelines around storage and use reinforce MOPS’ role in trustworthy science.
Every solution poured and every culture dish filled reveals unseen stories—the care and intent behind each buffer matters. Safety practices reinforce confidence, as handling MOPS requires gloves and good ventilation, tried-and-true habits in any lab. This substance meets safety and compliance standards certified by organizations like USP and FDA, which keeps trust high.
Labs now focus on efficiency and greener practices. Many aim to reduce waste and handle chemicals responsibly. MOPS’ high stability stretches its shelf life, which cuts down on unnecessary disposals. Labs explore ways to recycle containers and minimize single-use plastics when handling this buffer. Teaching students to respect both the chemistry in the bottle and the bigger environmental picture makes each buffer, including MOPS, a lesson in care for both data and planet.
Chemical formulas like C7H15NO4S don’t just sit on a shelf—they power breakthroughs, discoveries, and daily lessons in patience and detail. Getting the mixture right, down to the last atom, builds trust in every result and keeps the march of progress moving.
Sitting in a busy lab environment, it’s easy to let basic routines turn into afterthoughts. Chemicals land on shelves, sometimes tucked beside lunch boxes and coffee mugs. The reality is: poor storage habits don’t just risk wasted money—they set the stage for failed experiments, accidents, and a real mess. With something like 3-morpholino propanesulfonic acid (MOPS), taking shortcuts means you lose out on both efficiency and safety.
During my time in university research, I watched new grad students scramble to find reagents. Few things slow work like an unlabeled bottle or a container stored in the wrong place. MOPS, used often as a buffering agent, is sensitive to moisture and heat—conditions that sneak up fast in a crowded lab. I’ve seen bottles cake up, powders clump, and even changes in buffer strength. These changes undermine results and slow down projects that can’t afford repeat errors.
Let’s zoom out from imperfect memories and put facts on the table. MOPS has a solid track record in biological research because of its stability—when you treat it right. Exposure to open air brings moisture that damages its structure. A dry, cool place stands out as the gold standard for storing this acid. Researchers from Johns Hopkins and Stanford emphasize these types of practices in their lab manuals for a reason: humidity, even small amounts, triggers degradation over time. Heat also accelerates breakdown. A shelf near a radiator does more harm in a few weeks than a year spent in a temperature-stable cabinet.
The first habit I encourage: always seal your original packaging tightly. This sounds simple, yet misplacing caps or leaving bottles askew are the leading causes of spoiled chemicals. Use desiccators for longer storage, as they cut down on humidity. Most labs rely on polyethylene or polypropylene containers. Keep all stock bottles clearly labeled with purchase date or date you opened it. A label might sound dull, but tracking shelf life often catches supply chain problems before they ruin an experiment cycle.
If the space isn’t already climate-controlled, choose a cabinet or drawer away from sunlight and away from regular heat sources like radiators or computers. Avoid stacking rows upon rows—air needs to circulate. Those forced into makeshift spaces sometimes stash their bottles near fume hoods or even on random open benches. I once watched a coworker return after a weekend to find his reagent had taken up the scent—and possibly contamination—from a neighboring solvent bottle. Cross-contamination robs time and money.
Every careful step—resealing, labeling, smart positioning—acts as a safeguard for both your chemical and your lab’s results. MOPS isn’t expensive by lab standards, but every wasted gram adds up. Repeat mistakes in storage signal carelessness. And in labs, carelessness never just stops at one chemical. Building the habit of storing properly, based on manufacturer guidance and proven protocols, signals a respect for both science and teammates.
In the end, effective storage comes down to discipline. The labs I admired most weren’t necessarily the biggest or richest. They paid attention to the small details, trusted in proven storage habits, and stayed accountable.
Labs often rely on strong and predictable buffering systems, and 3-Morpholino Propanesulfonic Acid, or MOPS, is part of many scientists’ daily work. Its pKa value stands around 7.2 at 25°C, lining up with physiological pH and making it a favorite for many biological applications. I remember setting up protein purification experiments, and using MOPS helped keep my results consistent and my samples happy.
Buffer selection matters more than most admit. Swinging pH levels spell disaster for sensitive enzymes or protein conformations. The dependable pKa of MOPS draws biologists who need stability between pH 6.5 and 7.9. Its zwitterionic nature means less interference with biological reactions, which keeps surprises to a minimum. In practice, buffer choice ends up being the subtle difference between a successful blot and a wasted afternoon.
I've witnessed how a mismatched buffer wipes out hours of hard work. If your system operates near pH 7, straying from MOPS to something with a distant pKa—take glycine or acetate—doesn’t end well. Those buffers lose their grip near neutral pH, and the results grow unreliable. Using MOPS means the chemistry aligns with our needs, and the science gets clearer, not muddier.
This reliability encourages good science, the kind that can be trusted. Reproducibility remains a major problem in labs. According to a 2016 Nature survey, over 70% of researchers failed to reproduce others' experiments. I doubt all those issues came from buffers, but I’ve seen many troubleshooting sessions start with a sharp eye on buffer selection. The right pKa goes further than most people realize.
MOPS maintains its buffering action tightly around its pKa. Tiny movements in pH generate minimal change in charge, so proteins and cell cultures thrive with little unexpected side chemistry. Unlike phosphate-based buffers, MOPS won’t encourage metal precipitation or interact with cofactors in reactions. Sulfonic acids like MOPS carry low UV absorbance, which means spectrophotometry runs cleaner, with fewer background headaches.
Anyone can claim a buffer works well, but the research backs this up. MOPS appears in countless protocols for running SDS-PAGE, preserving cells, and supporting enzyme assays. Sigma-Aldrich, Merck, and major reagent providers list pKa values for MOPS right around 7.2, confirming the consensus. PubChem and chemical handbooks echo this number time and again. This figure gives scientists a practical target, instilling trust that runs deeper than the bottle label.
To avoid costly mistakes, researchers could double-check buffer tables before opening any container. Keeping a reliable reference list for buffer pKa values handy, especially for new students or rotating lab members, helps dodge the classic pitfalls. Training goes a long way too—guiding newcomers to pick buffers suited for both their experiments and their instruments. Automated pH meters do their job, but understanding why the pKa sits where it does brings the knowledge full circle.
Commercial kits often simplify things by pairing the right buffer to the correct assay, but this approach can separate users from the science. Sticking with well-documented choices like MOPS builds understanding and sharpens experimental intuition. Whether you’re troubleshooting tricky gels or raising a sensitive culture, picking a buffer with the right pKa makes science less of a guessing game and more of an exact pursuit.
Labs use all sorts of chemicals every day, and 3-Morpholino Propanesulfonic Acid, often called MOPS, pops up frequently as a buffer. Some hear “acid” and immediately worry. Truth is, you can’t figure out risk by name alone. The first question I always ask is the same one anyone should: what happens if I spill this on myself, breathe it in, or pour it down the sink?
Talking about MOPS, it doesn’t show up on lists of notorious toxins or acute poisons. There’s no big hazard warning taped to its bottles in reputable labs. Major suppliers share material safety data sheets (MSDS), and these always mention common risks—irritation for skin or eyes, stomach problems if you swallow it, or coughing if you breathe its dust. No one can ignore those. Lab safety basics matter for any chemical, and MOPS draws a line under that. Wear gloves, keep goggles handy, avoid splashing or tossing powder around.
I’ve measured out MOPS for cell culture experiments and cleaned up a few drops more than once. No dramatic stories to tell. MOPS works mostly in small amounts, gets diluted fast, and isn’t volatile. Even so, complacency brings trouble. Studies haven’t found MOPS causing long-term harm, but it’s wise to stay careful. Chronic exposure data is limited, so no one should treat any chemical as fully harmless just because huge risks don’t show up in everyday use.
Dumping leftover lab solution down the drain used to be common, but that has changed. Environmental policies grew tighter for good reasons. MOPS holds up well in water but can break down given time and the right conditions. Regulatory groups like the European Chemicals Agency highlight that excessive release into waterways might not be the best plan, hinting at problems for aquatic organisms at high concentrations. While I’ve never seen it listed as a major water pollutant, small actions stack up. Responsible disposal keeps MOPS—and every other buffer—from becoming tomorrow’s headache.
Good lab habits make the biggest difference. Staff at my old lab set up clear instructions for every chemical. New bottles got labeled fast. Old stuff, especially if it collected dust, went through proper waste programs. If you’re handling anything unfamiliar—including MOPS—read the MSDS, ask questions if something seems off, and never assume yesterday’s rules still apply. Regular safety training caught issues before they turned into incidents. Even seasoned scientists benefit from the occasional refresher.
Suppliers, too, serve a role beyond providing chemicals. Detailed safety information, training webinars, and quick response lines during mishaps build confidence for people working with all sorts of compounds. Policies requiring suppliers to post clear hazard ratings and disposal advice would help even more. That’s the sort of transparency every lab needs.
Science keeps moving fast, and buffer chemicals like MOPS support research all around the globe. Still, real safety depends on habits, respect for the unknown, and good cleanup after every experiment. MOPS won’t turn into a crisis for most people who take basic care, but it deserves the same attention as any chemical with an “acid” tag on the label. Better training, clear labeling, and thoughtful waste management keep science safe and progress steady.
| Names | |
| Preferred IUPAC name | 4-morpholin-4-ylbutane-1-sulfonic acid |
| Other names |
MOPS 3-Morpholinopropanesulfonic acid 3-(Morpholin-4-yl)propane-1-sulfonic acid |
| Pronunciation | /θriː-mɔːrˈfoʊlɪˌnoʊ proʊˈpeɪnˌsʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | “1132-61-2” |
| 3D model (JSmol) | `3Dmol:'CC(CS(=O)(=O)O)N1CCOCC1'` |
| Beilstein Reference | 1776119 |
| ChEBI | CHEBI:40337 |
| ChEMBL | CHEMBL414372 |
| ChemSpider | 85048 |
| DrugBank | DB11299 |
| ECHA InfoCard | 100.046.942 |
| EC Number | 1132-61-2 |
| Gmelin Reference | 1370738 |
| KEGG | C02338 |
| MeSH | D017928 |
| PubChem CID | 10346 |
| RTECS number | TY8050000 |
| UNII | J4U763I581 |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | 3-Morpholino Propanesulfonic Acid CompTox Dashboard ID: "DTXSID6015113 |
| Properties | |
| Chemical formula | C7H15NO4S |
| Molar mass | 205.26 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.211 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.7 |
| Vapor pressure | 1.47E-7 mmHg at 25°C |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 5.0 |
| Magnetic susceptibility (χ) | -62.2·10^-6 cm³/mol |
| Refractive index (nD) | 1.462 |
| Dipole moment | 5.22 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 190.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -499.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1494 kJ/mol |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 122°C |
| Lethal dose or concentration | LD50 oral rat 5200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5,000 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m3 |
| Related compounds | |
| Related compounds |
MES HEPES TES CHES PIPES |