People working with organic molecules have a knack for finding value in odd compounds, and 3-amino-1-propanesulfonic acid—often known as APSA or taurine analog—reflects this curiosity. Researchers set eyes on it in the early twentieth century. Back then, scientists, led by the surge of new inorganic syntheses, were on the lookout for water-soluble, functional molecules. Chemists like Hofmann and Strecker played a role by developing pathways for sulfonic-acid derivatives. APSA didn’t make it to global headlines overnight, but as analytical techniques grew more reliable, folks started recognizing its use as a zwitterion, balancing both acidic and basic properties, which started shaping its roles in research and applied chemistry. As decades passed, APSA slipped into the standard toolkit for biochemical analysis, dye production, and as an intermediate in synthesizing more complicated molecules.
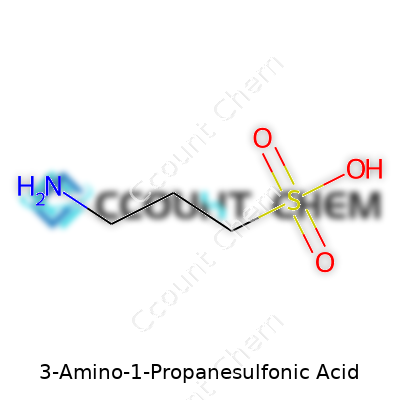
A look at the molecular structure gives away a lot. 3-Amino-1-propanesulfonic acid is a small organic compound, showing both an amine (-NH2) and a sulfonic acid (-SO3H) group, separated by a three-carbon chain backbone. That specific design brings about an interesting balance—it behaves a lot like taurine (common in energy drinks and animal tissues), but the structural shift opens up new reactivity. Its highly polar nature and readiness to form stable salts with both acids and bases make it a handy material for chemical synthesis, buffering systems, and industrial-scale reactions.
Anyone who’s handled APSA spots a crystalline white powder, completely odorless and fairly innocuous at first glance. Under room conditions, it stays dry and free-flowing. As a polar zwitterion, it dissolves easily in water, offering a clear solution across a broad pH range. It resists breakdown at room temperature and generally only starts to decompose above 200°C, emitting sulfur oxides and nitrogen compounds as it goes. Its molecular formula, C3H9NO3S, sits neatly with a molar mass hovering near 139.18 g/mol. Notably, it doesn’t mix well with non-polar solvents like hexane or benzene, so most of its uses stem from this water-soluble behavior.
In a laboratory or production setting, specifications drive reproducibility. Good batches of APSA claim purity levels above 99% on assay, minimum sulfate and nitrite impurities, controlled moisture contents, and a clear melting profile. Certified suppliers make sure to label the product with batch, expiration date, and origin, meeting labeling laws from agencies like the Food and Drug Administration, European Chemicals Agency (ECHA), and China’s Ministry of Agriculture. This careful detail on packaging, along with hazard and handling instructions, comes from lessons learned through decades of both mishaps and quality breakthroughs in fine chemical distribution.
The main route starts with 1,3-propanesultone, which reacts with ammonia or an amine under controlled temperature and pressure, delivering APSA in high yield. Some labs skip to sulfonating 3-aminopropanol, targeting more direct conversion. Others still lean on older methods, like the ammonolysis of 3-chloropropanesulfonic acid, but handling chlorinated starting material causes headaches with waste disposal. Practical synthesis means controlling by-products and limiting reactivity with atmospheric moisture, plus using solvents and reactors that withstand sulfonic acid’s corrosive potential. Recovery and purification steps—usually through recrystallization or ion-exchange resins—transform crude reactions into high-purity final material.
APSA stands as a chemical chameleon due to its two reactive ends. The sulfonic acid group attaches easily to metals and polymers—crucial for forming resin cross-linkers, anti-static agents, and dye intermediates. The amine group, no slouch either, welcomes alkylation and acylation, opening doors for modified amino acids and specialty surfactants. Chemists sometimes run cyclization reactions, building on APSA’s backbone to generate rings and bioactive molecules. In polymer chemistry, grafting APSA onto backbones improves water absorption or imparts charge properties that make specialty membranes or gels. These tweaks demonstrate how a fundamental understanding of functional groups makes or breaks industrial or medical applications.
Whoever’s hunted through catalogues for APSA quickly finds a laundry list of names: 3-aminopropanesulfonic acid, β-aminoethanesulfonic acid, 1-propanesulfonic acid-3-amine, and, less commonly, homotaurine. Global markets also recognize codes such as CAS 56-90-4. The proliferation of names stems from language barriers, application drift, and shifts in chemical nomenclature over decades. It pays to check labels carefully because these synonyms, while referring to the same backbone, might pop up with different purity standards or formulations.
Safety conversations don’t get enough attention in busy labs, but APSA demands the proper gear and handling. Its powdered form poses moderate dust hazards if inhaled, and like many sulfonic acids, skin contact occasionally brings about irritation or allergic response. Long-term exposure data remains limited, underscoring the need for gloves, eyewear, and dust masks. Regulators place it outside major hazard categories—for now—due to its low acute toxicity. Proper storage in sealed containers, away from oxidizers or bases, guards against both material loss and unpredictable reactions. Disposal runs through approved chemical waste streams. The Material Safety Data Sheet walks through all expected hazards and antidotes, and regulatory frameworks, from REACH to OSHA, circle back to common-sense precautions in workflow.
Certain compounds, like APSA, earn their keep by showing up where more specialized molecules falter. In textile and dye industries, APSA’s structure gives it edge as a coupling agent, binding colors more tightly to fibers and resisting fading. Water treatment outfits build on its chelating properties, using APSA to sequester heavy metals or balance ion-exchange resins. Analytical chemists lean on APSA as a buffering agent in high-performance liquid chromatography (HPLC) and other separation techniques because of its stable, predictable charge profile. In medical and life science research, APSA and its analogs form the skeleton for neurotransmitter studies, osmolyte replacement therapies, and drug metabolite research. Every time someone builds a new diagnostic kit or biofunctional polymer, there’s a fair chance they looked into tweaks based on this backbone.
R&D teams treat APSA as a scaffold—anchoring, modifying, and extending it in the hunt for new catalysts, pharmaceuticals, and smart materials. In the past decade, papers poured in from groups exploring APSA-derived molecules for neuroprotection, memory enhancement, and even as anti-amyloid agents to fight neurodegenerative diseases. Bioconjugate chemistry makes use of APSA’s dual ends, customizing diagnostic probes and nanocarriers. Synthetic chemists design greener production processes, focusing on solvent reductions, catalytic efficiency boosts, and waste minimization. The electric vehicle and fuel cell boom opened new lines of inquiry, with APSA-based membranes or electrolyte systems showing promise thanks to their hydrophilicity and robust tolerance to harsh environments. Universities and private labs both invest heavily in mapping these application landscapes.
Though APSA wins points for low acute toxicity in standard animal models, deeper reviews keep rolling in. Some studies suggest that homotaurine derivatives cross the blood-brain barrier more easily than taurine, which raises both hope and caution for pharmaceutical developers. Chronic exposure and ecological impact remain less defined, feeding into debates about accumulation or breakdown products in wastewater streams. Most available toxicity reports highlight mild irritation thresholds, but ongoing research pushes for more granular data—especially as some derivatives climb into pilot trial status for treating memory loss and neurodegeneration. Regulatory agencies encourage transparent handling, workplace exposure monitoring, and partnerships with academic toxicologists to limit unexpected headaches.
Innovation never leaves a chemically flexible molecule behind, and APSA’s journey seems far from over. Green chemistry pushes for more sustainable synthesis—using biodegradable solvents, biocatalysts, or even enzymatic pathways. Drug designers eye APSA analogs to treat epilepsy, cognition decline, or metabolic disorders, leveraging both targeting and improved brain-access profiles. Material scientists draw on its high solubility and ion-conductivity for next-gen smart hydrogels or battery membranes. The trend toward custom-made biointerfaces—a blend of polymers and biologically active agents—opens fresh terrain for APSA’s chemistry. As more research money shifts toward sustainable, high-functionality materials, the routines and protocols built around APSA offer a strong starting point. Regularly, new patents and studies bring APSA back into focus, not as an old staple, but as a launchpad for the next round of discoveries.
3-Amino-1-propanesulfonic acid, often called APS, slips under the radar for most people outside science labs. This compound pops up in discussions about buffer systems and specialty chemical manufacturing. Its profile rarely competes with crowd favorites like citric acid or sodium chloride, but it sits right where the fields of chemistry, biology, and industry overlap.
Lab workers bump into APS because it has just the right structure for building stable buffer solutions. When scientists run experiments that track how enzymes behave, shifts in pH can cause headaches. APS acts as a steadying hand, holding pH at a predictable level. Research I handled during a summer internship relied on buffers like these for reliable protein assays. Even a tiny shift in acidity could spoil a whole week’s worth of samples. APS’s compatibility with biological molecules and its moderate ionic strength made it the right fit for our project, especially when working with sensitive enzymes or cell cultures that cannot handle harsh conditions.
Researchers probing into neurological conditions have also found APS useful. Its unique structure allows it to mimic or inhibit some amino acids. This has helped laboratories test how nerves talk to each other or screen drugs that may adjust serotonin or dopamine pathways. Some animal models for epilepsy involve this compound because it triggers or blocks specific neural signals, helping scientists dissect how seizures start. I’ve read journal articles tracing key milestones in epilepsy research back to studies involving APS. This sheds light on how deep a small molecule’s impact can reach, even if most people never hear its name.
Industrial labs put this acid to work as a building block for synthesizing specialty chemicals. APS’s chemical structure makes it a good choice for adding sulfonic acid groups to new compounds. These sulfonated products often go into detergents or dyes. I remember touring a plant that made textile dyes, and engineers mentioned how these ingredients help pigments dissolve and stick to fibers. By tuning the process with a compound like APS, they improved product quality and cut costs from wasted batches. It gave me a look at how little tweaks in raw ingredients spill over into better, cheaper products people depend on every day.
Like many chemicals, APS comes with responsibility. Its sulfonic acid group means it dissolves easily, so any accidental release into water can travel quickly. Companies and labs handle storage carefully and train workers in safe disposal. Regulations often stand as guardrails so spills do not lead to bigger environmental headaches later. Student projects have tackled tracing sulfonic acids in local waterways, highlighting that even compounds used behind the scenes deserve close attention. Proper handling and disposal reduce risks for people and the planet. Good management protects labs and keeps surrounding communities safe.
APS shows how scientific progress often depends on parts most people barely notice. Clear labeling, up-to-date safety sheets, and training programs keep labs and factories running safely. Sharing data on environmental impacts with regulators and the public can push for safer uses and better alternatives. The world behind the scenes owes a lot to unsung players like APS, proving how the smallest building blocks still shape much larger stories.
3-Amino-1-propanesulfonic acid might sound like a mouthful, but it gives us a clear snapshot of both its structure and function. The molecular formula for this compound is C3H9NO3S. That formula tells a complex story for anyone who has opened a chemistry textbook. Picture three carbon atoms forming the backbone, nine hydrogens hanging off like streetlamps, and the all-important sulfonic acid group attached to one end. There’s also an amino group, giving the compound its unique character and uses in science and industry.
Some people may wonder why a formula like C3H9NO3S matters. In practice, this is not just an exam question or chemical trivia. The arrangement of atoms affects how molecules behave. If you throw two similar compounds into water or a living system, the outcome can change just because of a swapped group or a single extra hydrogen. The molecular formula helps researchers develop specific treatments, design safe laboratory processes, and even build new materials from the ground up.
Sulfonic acids, such as this one, catch the eye of researchers for more than one reason. Many are powerful acids in water and often form stable salts that resist breakdown. The amino group provides a site for further chemical reactions, which means it could get linked up or modified in making pharmaceuticals, specialty chemicals, or analytical reagents.
Back in my own studies, a formula like this gave me headaches until I started thinking about the real-world implications. Identifying molecules accurately prevents problems with lab work, clinical safety, and even manufacturing. I remember pulling an all-nighter before a final, working to memorize these basic formulas. In the years since, I’ve seen that this step isn’t just about passing a test—accuracy in such small things feeds into results you can trust.
A clear formula can point out potential hazards, too. The sulfonic acid group signals that this molecule falls in the family of strong acids (though still milder than sulfuric acid), so handling with care remains necessary. The amine group introduces concerns around chemical stability and interaction with other lab substances. If you don’t know what you’re dealing with, accidents happen. Everyone benefits when chemists can easily map a molecule right down to the atom.
Lab safety plans thrive on locked-down, unambiguous chemical identities. Using the right formula avoids mix-ups between compounds that might look or sound similar. For companies involved in chemical synthesis, accurate formulas save time and money. Mistakes early in a process can snowball into lost batches or worse—dangerous reactions.
Schools and universities could help by blending more real-world context with the tougher groundwork of chemical formulas. Instead of rote memorization, imagine students learning with hands-on models or problem-solving challenges. Having struggled with memorizing those formulas myself, I find it valuable when new learners see why precise chemical identities hold weight far beyond the pages of a textbook.
The story behind C3H9NO3S goes beyond carbon counts or matching test answers. Each detail of the formula supports safer labs, better products, and healthier outcomes down the line. In the world of chemistry, precision changes everything—and a simple formula keeps both science and industry on track.
Anyone who’s spent time in a chemistry lab or worked with chemical ingredients has run into the question of water solubility. Whether you mix chemicals for research, production, or curiosity, knowing if a compound dissolves in water has real consequences. For 3-amino-1-propanesulfonic acid, it’s no different. Chemists often need clear answers without jargon or confusion.
3-Amino-1-propanesulfonic acid, by its structure, carries both an amine and a sulfonic acid group. The amine group likes to grab onto hydrogen ions, making the molecule behave a little bit like an amino acid. The sulfonic acid group, on the other hand, features a highly polar structure that pulls toward water molecules. The whole molecule sits on short carbon backbone, making it small and easy for water molecules to surround. In practice, this results in a high level of solubility. This chemistry isn’t just theory—it’s what scientists observe when they try to make solutions in a beaker.
I remember working on a project in graduate school where we had to dissolve a variety of sulfonic acids in water. Some sulfonic acids with bulky side chains barely mixed, leaving cloudy residues in the flask. 3-Amino-1-propanesulfonic acid never gave such trouble. Shake it up, and the solid disappears into the liquid with ease.
The literature confirms what the lab shows. Published data lists 3-amino-1-propanesulfonic acid as freely soluble in water. Academic articles and supplier material safety data sheets specify this again and again. The reason is straight chemistry—the molecule is hydrophilic and does not resist water’s polar environment. Compared with longer-chain sulfonic acids or those with nonpolar side groups, this small compound cuts right through the worries of insolubility.
In industrial settings, manufacturing teams count on this trait. If a chemical adds cost or complexity to dissolve, it can throw off an entire process. In dye manufacturing, pharmaceuticals, and biochemical research, technicians want instant mixing. Time lost fighting insoluble powders costs labor dollars and compounds error. Water solubility in this case saves resources and keeps operations smooth.
Water solubility also signals how chemicals behave in the environment. In waste streams, soluble compounds tend to migrate readily, affecting how facilities manage disposal. Good solubility means 3-amino-1-propanesulfonic acid won’t stick around as residue in pipes or tanks. Instead, it travels with rinse water, urging operators to keep an eye on downstream treatment.
For those designing products, especially in the pharmaceutical and biotech fields, water solubility ensures active ingredients reach targets within the body. Human tissue and blood are mostly water, so if a chemical can’t dissolve, it can’t move efficiently through biological systems. With 3-amino-1-propanesulfonic acid, researchers get flexibility for new applications because dissolution hurdles don’t trip them up early in development.
While solvents and mixers can increase solubility in some tricky cases, there’s peace of mind knowing certain chemicals don’t need those extra steps. With 3-amino-1-propanesulfonic acid, users don’t wait for slow mixing, don’t need costly co-solvents, and don’t mop up stubborn sediment. This all reduces operational overhead and, from years on shop floors and in the lab, I’ve seen it take pressure off teams working under the clock.
Quality facts are never optional. Materials data must come from sources willing to show experimental numbers, peer-reviewed articles, and recognized handbooks. MSI data, Merck Index, and direct lab testing all show the same story—reliable water solubility for 3-amino-1-propanesulfonic acid.
I’ve spent years working in labs and supporting chemical research, and it’s never just about putting a bottle on a shelf. Every compound has its quirks. 3-Amino-1 propanesulfonic acid, often found as a powder or in crystalline form, wants respect on the storage front. People sometimes underestimate the way humidity, heat, or even just a forgotten container can lead to ruined experiments or, worse, workplace hazards.
Most places focused on materials safety will say, "keep it cool and dry," but I’ve seen eager newcomers miss what that means in the real world. Heat speeds up chemical breakdown. Run-of-the-mill room temperature storage feels harmless—until the thermometer creeps up or that storeroom AC quits over a holiday. In my experience, aiming for a steady, cool environment—ideally between 2°C and 8°C—keeps the compound stable longer. Refrigerators meant for chemistry, not for food, work best. Avoiding the temptation to use a kitchen fridge full of leftovers goes a long way in protecting integrity and preventing accidental contamination.
Moisture is a tough customer too. This acid loves to pick up water from the air, especially in humid climates. Once the powder cakes up or dissolves a bit, it’s easy to lose purity. I always use airtight containers made of sturdy materials. Glass jars with rubber seals or high-quality plastic with a tight screw cap will do the trick. I’ve seen desiccant packs save many batches—those little silica gel packets may not look like much, but they suck up stray humidity and spare you expensive repurchases.
Light gets missed in day-to-day handling, but UV or bright light has no business hitting most chemical powders for long stretches. It can start breaking down organic compounds. Opaque or amber containers prove their worth over time. I tell colleagues to shelve their 3-amino-1 propanesulfonic acid in low-light cabinets, away from windows or workspace lights. It doesn’t take much foresight, and it saves grief later.
Safe storage supports not just the stability of 3-amino-1 propanesulfonic acid, but also the health of everyone in the lab. Labels matter. I always mark containers with the chemical name, concentration, and the date they landed in storage. Mixing up reagents or grabbing a mystery bottle turns a routine task into a safety incident. Keep acids away from reactive substances—oxidizers, bases, or anything flammable. Separate shelves or bins cut down risks in busy environments, and it’s one of those habits that pays off in safety audits and day-to-day working life.
Poor storage isn’t just about wasted materials. Exposure to moisture or strong acids can corrode shelves, damage surfaces, and, worst of all, create chemical reactions that threaten air quality or personal safety. People have gotten sick or injured from mishandled substances, so diligent storage isn’t a suggestion, it’s a requirement. Regular checks, a little organization, and sticking to protocols protect both personnel and research outcomes in any lab setting.
Some chemicals spark worry just because their names sound intimidating. 3-Amino-1-propanesulfonic acid falls into that category. Chemists and manufacturers use it, but that doesn’t mean its dangers are widely understood. People want to know if it’s hazardous or toxic, especially since safety at work or in a lab is never just a box-ticking exercise.
You don’t need to be an industrial chemist to dig into scientific references. Toxicology databases, such as PubChem and ECHA’s registered substances page, put ingredient safety front and center. 3-Amino-1-propanesulfonic acid is not flagged as a significant hazard for humans under normal circumstances. My own digging led me to a range of sources, including chemist discussion forums where lab techs shared their everyday experiences. Over years of use, no one reported health emergencies unique to this chemical.
Manufacturers have not reported this acid causing cancers, genetic mutations, or reproductive toxicity in animal studies. Unlike more volatile or corrosive laboratory acids, it tends to stay put as a solid powder and doesn’t evaporate or burn skin instantly. Still, nothing in science is all-or-nothing.
Even chemicals with a mild track record aren’t toys. Inhaling powders or creating dust has always felt risky to me, no matter what the safety sheet says. That’s common sense made official by occupational guidelines; avoid breathing dust and keep it off the skin. Goggles, gloves, lab coats—these are habits, not just for “dangerous” substances but for every shift at the lab bench. Nobody regrets being “too careful” when an eye splash is on the line.
The acid’s sulfonic group doesn’t make it volatile, and its toxicity is lower than common acids like hydrochloric or sulfuric. I’ve seen people get red, irritated skin if they’re careless, but nothing lasting or severe. Standard first aid—washing the site and watching for a reaction—does the trick.
If this compound ends up in a major spill, the main worry centers on water contamination. Sulfonic compounds don’t break down quickly. Fish and aquatic life handle these additives poorly, so an accidental dump into a river could hurt the environment. That ties into a wider problem—too many mild chemicals build up and create chronic stress in nature.
Waste disposal rules may not cover every specific chemical. Some labs lump “mild” acids together, which leads to casual dumping. Better labeling and supervisor oversight make a difference. We also benefit from regular discussions about what goes down the drain. I’ve found that even seasoned lab workers skip over the environmental impact in fast-paced work.
Trust in clear labeling and honest Material Safety Data Sheets goes far. Anyone handling chemicals needs short, direct instruction—not pages of legalese. Quick access to eyewash stations and good housekeeping in the workspace always matter.
Chemistry doesn’t have to be mysterious for those outside the field. Maintaining respect for every ingredient, even quiet ones like 3-amino-1-propanesulfonic acid, avoids both overreaction and complacency. Staying alert, asking questions, and staying open about process tweaks helps everyone stay safer, from scientists to the communities living downstream.
| Names | |
| Preferred IUPAC name | 3-Aminopropane-1-sulfonic acid |
| Other names |
Homotaurine 3-Aminopropanesulfonic acid Aminoethylmethanesulfonic acid Amino-1-propanesulfonic acid Gamma-amino-1-propanesulfonic acid |
| Pronunciation | /ˈæmɪnoʊ waɪn proʊˈpeɪnˌsʌlˈfɑːnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 333-20-0 |
| 3D model (JSmol) | `3D Model (JSmol) String` for **3-Amino-1-propanesulfonic acid** (commonly known as Homotaurine): ``` CC(CS(=O)(=O)O)N ``` This is the **SMILES** string representation, widely used for JSmol and similar 3D modeling platforms. |
| Beilstein Reference | 1739753 |
| ChEBI | CHEBI:38845 |
| ChEMBL | CHEMBL12035 |
| ChemSpider | 65648 |
| DrugBank | DB08797 |
| ECHA InfoCard | 03b792013b02-40ee-b504-66cd346b198d |
| EC Number | 217-034-5 |
| Gmelin Reference | 35803 |
| KEGG | C01794 |
| MeSH | D017211 |
| PubChem CID | 15430 |
| RTECS number | TY1575000 |
| UNII | 7O67039P3P |
| UN number | UN3335 |
| CompTox Dashboard (EPA) | DTXSID7039527 |
| Properties | |
| Chemical formula | C3H9NO3S |
| Molar mass | 137.17 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.41 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | 1.78E-7 mmHg at 25°C |
| Acidity (pKa) | 1.63 |
| Basicity (pKb) | 5.35 |
| Magnetic susceptibility (χ) | -48.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.497 |
| Dipole moment | 6.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 161.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -221.2 kJ/mol |
| Pharmacology | |
| ATC code | N06AX13 |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P261, P264, P270, P271, P301+P312, P304+P340, P312, P330, P501 |
| Flash point | 83°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (oral, rat) |
| NIOSH | RU8925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.2 mg/m³ |
| Related compounds | |
| Related compounds |
Taurine Homotaurine Cysteic acid 2-Aminoethanesulfonic acid 1-Aminopropane-3-sulfonic acid |