For decades, chemists looked for ways to improve the efficiency and color stability of dyes. The roots of 3,4-dichloroaniline-6-sulfonic acid trace back to dye research in the early to mid-20th century, when improving textile dye processes became a national priority in many countries. German and Swiss chemical giants invested heavily in dye intermediates, out of necessity for vivid and lasting colors in an era when natural dyes couldn’t keep up with demand. This compound rose in importance whenever sulfonic acid groups were needed to boost solubility, and the dichloroaniline backbone provided reliable reactivity for further modifications. Over the years, chemists adjusted methods of synthesis using increasingly sophisticated technology, yet the core structure has remained largely the same. Seeing how persistent chemical discoveries shape our industries shapes my perspective on why understanding the material history matters.
3,4-Dichloroaniline-6-sulfonic acid supports modern production of advanced azo dyes, specialty pigments, and some agrochemicals. Known for its strong electron-withdrawing groups, the compound’s profile lends itself to critical roles in colorant chemistry. Manufacturers favor this molecule when seeking to introduce both high stability and favorable water solubility. Commercially, the product shows up as a crystalline powder or slightly off-white solid, with specialized companies tailoring purity and particle size for different industrial needs. It stands out for its balance between efficient manufacturability and adaptability in downstream reactions.
Solid at room temperature, 3,4-dichloroaniline-6-sulfonic acid offers predictable handling and storage. It dissolves well in water thanks to its sulfonic acid group, and less so in organic solvents—an advantage for aqueous processing and wastewater control. Chemically, it carries a molecular formula of C6H4Cl2NO3S and a molecular weight hovering around 258 g/mol. The dual chloro groups tighten its aromatic system, influencing both reactivity and toxicity. Its stability under moderate temperatures aids shipping and storage, though dust control and moisture management always deserve careful attention—something I learned quickly in a pilot plant setting, where a few misplaced scoops meant cleanup headaches and potential exposure risks.
Industrial buyers expect reliable specifications on assay, water content, and total chlorine. Typical labeling covers lot number, net weight, purity percentage, and manufacturer’s safety data reference. The product often ships in multi-layered bags or high-density polyethylene drums, with chemical names and hazard diamonds displayed plainly. Every detail counts—clean, correct labeling improves workplace safety and compliance with international chemical trade rules, an issue that caused serious delays for a manufacturer I once assisted during a regulatory audit.
The journey from raw chemicals to finished product follows a logical pathway. Chemists generally start with 3,4-dichloroaniline, introducing sulfonic acid functionality at the 6-position through a sulfonation reaction, commonly using oleum or chlorosulfonic acid under controlled temperatures. Reaction exotherms require strict process control. Purification involves multiple recrystallizations from water and filtration cycles to remove unreacted materials and colored impurities. Reactor design, agitation speed, and acid handling protocols drive batch consistency. At larger scale, automated feed systems and real-time analytics keep impurity loads low and yields up.
3,4-dichloroaniline-6-sulfonic acid serves as a versatile intermediate. Many chemists apply diazotization to the amino group, followed by coupling with aromatic compounds to build complex azo structures—dyes with intense color and strong fastness. Reduction and halogenation open pathways for pharmaceutical synthesis or custom derivatization. The sulfonic acid group improves solubility and functional group tolerance during multi-step synthesis, offering unique leverage in the chemist’s toolkit. Whenever downstream reactivity impacts final product quality or environmental profile, understanding primary intermediates like this one becomes crucial.
Chemicals often go by several designations, depending on origin and application. 3,4-dichloroaniline-6-sulfonic acid sometimes appears on labels as 2,4-dichloro-5-sulfamoylbenzenamine, or as its sodium salt in certain formulations. European suppliers use trade names tied to specific dye lines, though the essential chemistry remains the same. Familiarity with synonyms prevents double-ordering in multinational supply chains and ensures regulatory documents line up—nothing tests patience like customs holdup over poorly cross-referenced chemical names.
The compound scores moderate on the hazard scale. Direct contact irritates skin and eyes, and inhaled dust can trigger respiratory symptoms. Safety protocols dictate gloves, goggles, and well-ventilated areas for transfer or blending. Facilities set up emergency rinsing stations and maintain carbon filters in air systems. Material handling staff follow strict rules around chemical compatibility and fire safety due to possible generation of chlorine gas if mixed improperly. Environmental standards require capture and treatment of production effluents—regulators pay special attention to aromatic sulfonic acids due to persistent organic pollutant concerns. Everyone in the chain, from worker to transporter, must know storage incompatibilities and the right response steps, especially after a few near-misses led to improved emergency drill protocols at a site I once consulted.
Textile dye makers rely on 3,4-dichloroaniline-6-sulfonic acid for building direct and reactive dyes that hold up to repeated washing. Beyond coatings and fibers, pigment manufacturers tap into these intermediates for plastics, inks, and industrial paints. Agrochemical developers include it as a precursor in some herbicide and pesticide formulations, exploiting the unique electronic makeup for targeted biological activity. Water treatment experts investigate new uses, seeking improved oxidant delivery and trace pollutant removal. The compound plays a real-world role wherever controlled color, chemical durability, and biological properties must converge.
R&D laboratories continue to experiment with new reaction pathways, greener solvents, and catalysts to improve yield and lower waste streams. Computational chemists and process engineers collaborate to optimize reaction kinetics, aiming to limit side reactions and boost selectivity. Academics investigate structure-activity relationships, hoping to tweak the skeleton for even better properties in niche dye and drug molecules. Driven by international pressure for safer and more sustainable chemicals, companies invest in continuous-flow synthesis and alternative sulfonation protocols. My own experience in a startup lab taught me that breakthroughs rarely come from a single tweak; progress emerges over years of careful, documented trial and error.
3,4-dichloroaniline-6-sulfonic acid, like many dichlorinated aromatics, comes under chronic toxicity review. Researchers study animal exposure data, examining liver, kidney, and reproductive effects at different doses. Waste treatment specialists track breakdown products, monitoring for persistent residues in effluents. Regulatory agencies such as the US EPA and Europe’s ECHA investigate reports of long-term soil and water impacts. Comprehensive toxicity mapping shapes raw material choices and drives innovation in wastewater treatment, aiming to push contaminant levels below reporting thresholds. In my years with environmental monitoring crews, community safety and regulatory demands felt ever-present, especially in regions with a legacy of chemical runoff.
The chemical industry faces mounting pressure for transparency, traceability, and environmental accountability. Producers experimenting with bio-based aniline precursors, lower-waste synthesis, and closed-loop water systems look to lower the environmental footprint of supply chains. Adoption of automated handling and process analytics helps improve product quality and reduce exposure risks. Advances in green chemistry may replace certain raw materials or process steps, yet the core function of 3,4-dichloroaniline-6-sulfonic acid as a crucial dye and pigment intermediate remains secure for the foreseeable future. As techno-economic forces and sustainability requirements reshape the field, forward-looking manufacturers and scientists track both regulatory changes and customer demand for safer, cleaner material streams.
People working in dyes and pigments might give a little nod of recognition when someone mentions 3,4-Dichloroaniline-6-Sulfonic Acid. This chemical doesn’t get paraded in glossy brochures, but it's tucked deep behind the color we see in plenty of industrial and textile products. I’ve spent enough time talking with manufacturers to know its main job often connects directly to making raw materials that turn into pigments. These pigments help color fabrics, plastics, inks, and coatings, giving a visual punch that’s hard to miss, even if the compound itself isn’t flashy.
Most folks see the final product—a brightly colored shirt or a deeply pigmented plastic chair. Few realize how many steps the whole thing takes. 3,4-Dichloroaniline-6-Sulfonic Acid acts as a solid building block in that chain, especially in the synthesis of azo dyes. These dyes have a solid reputation for holding up under sunlight and washing. The chemical structure of this compound brings stability and intensity to the dyes. Its journey usually begins in a chemical reactor, handled by teams who track purity, toxicity, and reactivity, because safety and reliability matter more than anything in chemical production.
Regulatory frameworks tightly control anything tied to aniline derivatives. If you’ve ever had to wade through environmental oversight or strenuous safety audits, you know that mistakes can lead to big trouble—cleanup costs, ruined reputations, or worse. Aniline-based intermediates can break down into toxic components. 3,4-Dichloroaniline-6-Sulfonic Acid doesn’t escape tough scrutiny. Researchers report that accidental releases or improper disposal carry serious risks to aquatic life. Some labs have tracked its persistence in water sources, raising questions for communities near waste streams. Nothing commands attention like a regulatory agency with concerns in its reports.
I once talked with an environmental safety officer who stressed the strict rules on storing and transporting these materials. Even a minor spill sparks emergency drills into action. Chronic exposure to similar chemicals has shown troubling links to skin and respiratory problems. This puts extra weight on the need for gloves, goggles, and diligent handling. Companies that don’t respect this risk take a gamble with human health. The chemical’s breakdown products have drawn scrutiny, too, with studies flagging possible carcinogenic residues.
Fixing the problems tied to these industrial intermediates takes more than a warning on a storage tank. Some chemical engineers work on alternative reaction pathways, hoping for safer ingredients or less-hazardous byproducts. Green chemistry isn’t just buzz—it offers a way to get the desired pigment results with fewer environmental headaches. Investments in waste treatment help remove residues from water before they hit rivers and streams. Governments have started pressing for substitutes where possible, pushing industry toward options that leave less of a mark on people and nature. I’ve seen textile companies succeed with safer dye formulations, drawn by shifting consumer demand and export requirements.
Knowing what goes into synthetic dyes pays off for companies and customers alike. 3,4-Dichloroaniline-6-Sulfonic Acid turns up wherever colors matter. It sits at the crossroads of tradition and innovation, forcing periodic re-examination of safety, process, and impact. For anyone with a stake in manufacturing or environmental quality, real progress depends on an honest look at every chemical building block and the determination to do better when the evidence calls for it.
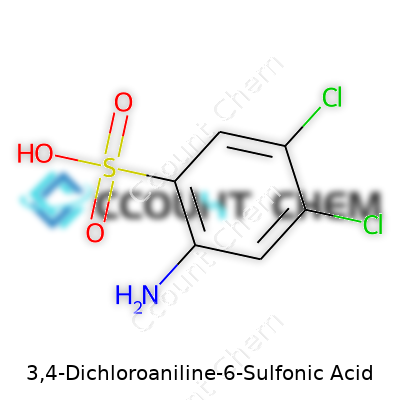
3,4-Dichloroaniline-6-sulfonic acid draws attention among chemists working in dye chemistry and specialty chemical manufacturing. Its chemical formula is C6H4Cl2NHSO3H. The structure builds on a benzene ring: two chlorine atoms hold the 3 and 4 positions, an amino group at the 1-position, and a sulfonic acid group crowds the 6-position. The arrangement of these groups shapes its usefulness and the challenges that come with handling it.
Putting two chlorine atoms on the aniline ring changes not just the reactivity. It tunes the electronic properties and, by extension, the solubility and stability. Sulfonic acid groups increase water solubility, which matters a lot in synthetic dye processes. The chemical stands out for its role as an intermediate, especially in the syntheses of certain azo dyes and pigment-related products. In all these cases, the specific positioning of each functional group determines if the molecule reacts as needed or just causes headaches in the plant.
Handling this compound safely means knowing both the risks and the practical uses. The sulfonic acid makes it more water friendly, but the chlorines mean you’re dealing with something far from benign. I’ve seen plant operators take extra steps with gloves and fume hoods, because exposure can trigger irritation. Low-level, chronic exposure to related chlorinated aromatic compounds links to health concerns, not to mention the environmental persistence if leaks happen.
Dye houses and pharmaceutical manufacturers use intermediates like this for reliable results. The presence of chlorine and sulfonic acid in the same molecule makes it particularly valuable in synthesizing fast-color, water-soluble dyes. Textile industries don’t compromise on colorfastness, so reproducible chemical structure counts for a lot. Every batch begins with compounds like this, and quality control starts right at the chemical’s source.
Working in chemical research, I’ve seen how older processes sometimes let too many chlorinated byproducts slip through. This increases environmental load. Efforts to reduce these emissions don’t just keep regulators happy. Neighbors and downstream users care, too. Large-scale manufacturers already explore alternative syntheses to cut down hazardous waste. Closed-loop water systems and upgraded scrubbers now help trap and neutralize toxic components before they ever hit the environment.
There’s growing pressure on chemists to replace or minimize the use of persistent, toxic compounds. Academic labs and industry teams put plenty of hours into designing greener analogues—sometimes shifting away from chlorinated aromatics altogether or using mild reaction conditions that make less waste. These aren’t just trend words but daily realities for research chemists and production engineers who must balance performance, safety, and environmental pressures.
Understanding the formula and function of 3,4-dichloroaniline-6-sulfonic acid pays off in both consistency and safety. Open discussion about its risks—paired with real efforts to improve handling and disposal—makes a real difference. It’s not only about what goes on in the beaker or the vat, but how practices ripple out into the wider world.
Factories across the world use 3,4-Dichloroaniline-6-Sulfonic Acid to create dyes for fabrics, plastics, and inks. Most people never hear its name, but it plays an everyday role behind the scenes. Sitting at a chemistry bench back in college, I learned that just because a chemical does its job in industry doesn’t mean it’s harmless. Handling even a beaker of such stuff made my gloves crinkle, and for good reason.
Studies carried out in Europe and Asia have shown that similar compounds often stick around in soils and waterways longer than expected. Even small spills or leaks from manufacturing plants or improper disposal can leach these chemicals into the environment. Tests on fish and water plants highlight trouble: certain sulfonic acids stress aquatic life, stunting growth. Direct contact on skin may cause irritation. Breathing in dry powder or fumes isn’t wise either, as researchers have linked exposure to nausea and sore throats.
Some people shrug off chemical hazards as problems only for scientists. Working a summer job at a textile dying facility, I watched coworkers with raw, red hands, even though gloves were mandatory. After a shift, washing up never quite stopped that chemical smell. Sooner or later, health effects catch up. Eye irritation, headaches, patches of rash—the body finds ways to warn us.
At the community level, run-off and accidental spills near rivers affect more than just manufacturing teams. Farmers downstream might see stunted crops, kids playing near creeks might break out in a rash, and local wells can suffer. Animal and environmental health get tied directly to how responsibly these compounds are handled.
Basic safety gear like gloves and masks help, but some companies cut corners. Regulations in developed countries force safer storage and disposal, but factories in other regions sometimes dump waste without treatment. Installing water filters and better ventilation costs money up front, but these upgrades prevent expensive cleanup and lawsuits later. Community monitoring groups, often organized by volunteers, keep a closer eye on suspicious dumps or smells.
Waste treatment can break down sulfonic acids into safer forms. Soap-and-water scrubbing in an emergency slows down exposure on skin, but training and information go further. Factories that share hazard data and encourage workers to report spills build trust and long-term safety. Engineers and chemists keep developing alternatives that do the same job while breaking down faster in nature.
I’ve come to see chemical risk less as an abstract story of hazard scores and more as a chain of choices—by companies, regulators, workers, and neighbors. Avoiding short-term profits in favor of common-sense safety pays off. Every time a safer substitute gets used or a spill is cleaned quickly, it’s an extra layer of protection. Staying curious, asking questions, and insisting on transparency protect everyone.
3,4-Dichloroaniline-6-Sulfonic Acid isn’t something you want to leave lying around without a plan. It’s a specialty chemical often involved in dye manufacturing and pharmaceutical processes. With a background in lab safety, I’ve learned the hard way that skipping steps or letting your guard down can mean disaster, especially with chemicals less widely known outside advanced manufacturing circles.
Keep this compound in a cool, dry spot. It reacts with moisture and might degrade over time if left out in the open or exposed to fluctuating temperatures. Many small-scale operations rely on basement or garage spaces, but humidity and summer heat can creep in. Control the room temperature with HVAC units or dedicated refrigeration if needed, especially in hot regions. Consistency pays off not only for stability but to lower accident risks as well.
This is a powder that stains and irritates. I’ve seen corroded shelving and sticky spills just from careless handling—not to mention the headaches if it creeps onto your skin or gets into your lungs. Go for solid, sealed containers made from materials that won’t react or break down, like high-grade plastics with tight-fitting lids or coated stainless steel. Label everything clearly in readable print and use secondary containment trays. Old jars and mismatched lids won’t cut it here. Organize by shelf—not just by type—keeping incompatible substances far apart. This is where a spreadsheet or detailed log comes in handy, especially once a collection of chemicals begins to grow.
This compound doesn’t belong anywhere near food or drink. Even shared spaces like break rooms and janitor closets should stay off-limits. If children or untrained staff have access to the building, store the acid in a locked cabinet or behind keypad entry. OSHA recommends these barriers for a reason. Curious hands or a simple mix-up could cause a serious emergency—don’t rely on warning signs alone.
When moving or handling the acid, proper personal protective equipment sets the groundwork for safety. Lab coats, splash-rated goggles, and heavy gloves have saved me and my colleagues countless times. Respirators matter if dust might get airborne, even in small quantities. Don’t knock over containers or try to quickly “clean up” a spill without trained backup and a spill kit ready.
Local rules dictate disposal and storage limits for chemicals like this, so check the Environmental Protection Agency and municipal guidance. Sometimes the simplest close call, like an unexpected leak, leads to a costly cleanup or even a fine. Never pour this or any residue down the sink—it gets into water systems and stays for years. Collection by certified hazardous waste professionals protects the groundwater, employees, and local wildlife.
Safer chemical management starts with training. Even if you’ve worked with chemicals for years, refresh your team with annual walkthroughs and hands-on drills. Update storage conditions with regular checks for leaks, expired containers, or damage. Storage upgrades pay for themselves over time since one single accident or inspection failure brings larger problems. Above all, respect the science—storage rules aren’t red tape but decades of experience written into practice.
Anyone who’s worked in a modern chemistry lab can recognize the need to quickly identify a compound by sight. In the case of 3,4-Dichloroaniline-6-Sulfonic Acid, you’re usually dealing with a powder or crystalline solid. Its color tends to fall in a pale to light yellow range. Sometimes, a slight beige tint sneaks in, especially if the batch hasn’t been purified thoroughly. The grain of such compounds isn’t just about the chemistry behind them—physical form can impact how a chemist stores or weighs a material. Dusty textures create handling hassle, and a uniform crystalline appearance makes life in the lab easier. Using a spatula to scoop out the acid, you notice no odor, which stands in sharp contrast to many aniline derivatives. That already tells you a little about the potential safety profile.
No one spends much time in a synthesis lab without learning how solubility shapes the path forward. 3,4-Dichloroaniline-6-Sulfonic Acid mixes well with water. This trait matters deeply—pairing the sulfonic acid group with those chlorine atoms brings up the water solubility pretty high, despite aniline’s reputation for resisting dissolution. You’ll often see clear, almost colorless solutions forming after a gentle stir, meaning you’re not fighting to get it into solution like you do with other less polar organics.
Organic solvents, on the other hand, give a different story. Try adding the acid to ether, chloroform, or nonpolar solvents, and frustration soon follows. There’s little to no solubility in these, showing the power of the sulfonic acid group to keep the molecule polar. This means you can separate the compound from nonpolar impurities during purification, a real-world advantage for chemical manufacturers and lab techs. Think about the waste streams too—using water as a solvent helps reduce dependency on harsher, more toxic solvents. That carries safety and sustainability advantages over time.
From my experience, the appearance and solubility traits guide decisions early on. The pale, crystalline texture lets a chemist check quality at a glance and recognize potential contamination or breakdown. Solubility—especially in water—shapes a lot of choices. Waste streams can be simpler to treat, since water-soluble materials can be diluted and neutralized more safely. Workplace safety takes a turn for the better too, since water solubility reduces the risks of inhaling dangerous dusts or splashes during cleanup. In a packed industrial operation, these straightforward differences drive safer shifts and more predictable processes.
One persistent issue involves separating the compound from water after a reaction. Getting high-purity crystals back out without introducing new hazards or residues always turns into a balancing act. Skilled chemists use a careful touch, relying on controlled cooling and slow evaporation for better yields. At scale, efficient filtration and recovery systems chip away at losses and help prevent byproducts from slipping into the environment.
Curiosity about solubility and physical form isn’t just a matter for chemical engineers—regulatory compliance and long-term environmental safety both need answers too. Using water-based systems can encourage greener chemistry initiatives, addressing concerns about volatile solvent use. Research into innovative purification setups—like membrane filtration or advanced crystallizers—aims to improve isolation and reduce water waste even further.
Every unique compound brings a fresh set of opportunities and pitfalls. 3,4-Dichloroaniline-6-Sulfonic Acid’s pale color, powdery texture, and strong water solubility shape both daily lab life and big-picture decisions in production. Recognizing these traits right away keeps teams on track and sets a solid foundation for better processes in the future.
| Names | |
| Preferred IUPAC name | 4,5-Dichloro-2-aminobenzenesulfonic acid |
| Other names |
2,5-Dichloro-4-aminobenzenesulfonic acid 3,4-Dichloro-6-aminobenzenesulfonic acid C.I. 37190 intermediate C DCSA |
| Pronunciation | /ˈθriː,ˈfɔːr-daɪˌklɔːr.oʊ.əˈnɪliːn-sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-63-1 |
| 3D model (JSmol) | `3d_7b31b4e4b0e830f2bbf5a89e0e31c178` |
| Beilstein Reference | 1518985 |
| ChEBI | CHEBI:136265 |
| ChEMBL | CHEMBL1748505 |
| ChemSpider | 16838630 |
| DrugBank | DB14603 |
| ECHA InfoCard | 100.010.248 |
| EC Number | EC 219-937-1 |
| Gmelin Reference | 7664 |
| KEGG | C07279 |
| MeSH | D017350 |
| PubChem CID | 122323 |
| RTECS number | DJ0400000 |
| UNII | 1D7Q1E1RNH |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C6H4Cl2N2O3S |
| Molar mass | 261.08 g/mol |
| Appearance | Light yellow to gray white powder |
| Odor | Odorless |
| Density | 1.78 g/cm³ |
| Solubility in water | soluble in water |
| log P | 0.89 |
| Vapor pressure | 0.000119 mmHg at 25°C |
| Acidity (pKa) | 1.53 |
| Basicity (pKb) | 6.5 |
| Magnetic susceptibility (χ) | -0.41 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.658 |
| Dipole moment | 3.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 236.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -679.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1329 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P280-P261-P305+P351+P338-P302+P352-P304+P340 |
| Flash point | > 230°C |
| Lethal dose or concentration | LD50 oral rat 1470 mg/kg |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (oral, rat) |
| NIOSH | Not established |
| PEL (Permissible) | PEL (Permissible exposure limit) for 3,4-Dichloroaniline-6-Sulfonic Acid is not specifically established by OSHA. |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
3,4-Dichloroaniline 4-Chloroaniline-2-sulfonic acid 3,4-Dichlorobenzenesulfonic acid 3,4-Dichlorophenol 2,4-Dichloroaniline 3,5-Dichloroaniline 4-Nitro-3,5-dichloroaniline 3,4-Dichlorotoluene |