The journey of synthetic antifungal compounds, marked by the advent of triazole chemistry, reshaped approaches to infectious disease. Research shifted from basic azoles and moved into more specialized triazoles as microbiologists recognized how subtle chemical changes could dramatically affect activity against resistant fungal strains. By the early 2000s, medicinal chemists looked with sharper focus at side chains and ring modifications, hunting for that sweet spot between human safety and pathogen lethality. After long stretches of lab trial and error, compounds like (2R*,3S*)-2-(2,4-Difluorophenyl)-3-(5-Fluoro-4-Pyrimidinyl)-1-(1H-1,2,4-Triazol-1-Yl)Butan-2-Ol emerged. Camphorsulfonic acid salt forms improved solubility and handling, opening new doors for both research and production. I remember poring over stacks of journals to understand how a single added fluorine atom turned an ordinary antifungal backbone into something more potent and selective—a true testament to the power of methodical synthesis combined with hard data from international collaborations.
You’ll find this compound sitting at an intersection where pharmaceutical ambitions meet the raw needs of hospitals dealing with invasive mycoses. The molecule bridges key classes: triazoles for their broad-spectrum fungicidal action, camphorsulfonic salts for pharmaceutical stability. Researchers favor this salt for better crystallinity as well as manageable hygroscopicity, standing out amid cluttered shelves of unstable, finicky azole powders. As someone who’s worked with both pharma startups and academic labs, fewer problems in basic handling translate to shorter development times, fewer wasted resources, and absolute clarity in reactivity. Supply chains that carry this compound usually stock it for high-value antifungal R&D, sometimes for use as a reference standard in preclinical and even clinical stages.
Take a look at the white or slightly off-white crystalline powder. With a melting point generally in the range suited for solid oral formulations, it resists the kind of moisture uptake that can turn other triazole salts into a sticky mess after a month in the wrong climate. The compound’s molecular architecture—thanks in part to those fluorines—strengthens resistance to metabolic breakdown. Solubility in common solvents like DMSO, ethanol, and water (under certain pH conditions) gives formulators options. Under UV, the pyrimidinyl and triazolyl groups provide reliable spectroscopic signals, simplifying purity assays. High performance liquid chromatography often shows a single strong peak, testament to synthesis pathway efficiency and strict quality control. Analytically, the camphorsulfonate moiety doesn’t interfere with standard detection methods, which simplifies interpretation for those in R&D or regulatory work.
Regulatory filings demand precision: each vial is labeled by batch, storage requirements (usually 2–8°C, protect from light), net content, and molecular formula. Technical sheets list the expected appearance, melting range, percent purity—often above 98% by HPLC—alongside key spectral data (proton NMR, IR fingerprints, mass spec confirmation). Any residues, such as solvents or acids from synthesis, get flagged with strict thresholds: pharmacopoeial standards tolerate no room for error. Barcode-driven batch tracking allows quick response if a quality issue emerges. MSDS documents show recognized hazards—mostly skin, eye, and inhalation irritancy—for both the salt and parent compound. My own experience always says: double check every technical label, especially the storage instructions, if you want reproducibility in your own lab.
Chemists typically approach synthesis in multistep fashion: a central triazolylbutan-2-ol backbone forms first by coupling an epoxide or halohydrin precursor with triazole nucleophile, demanding careful control of temperature and polarity to avoid byproducts. Next, selective fluorination of phenyl and pyrimidinyl rings employs reagents such as N-fluorobenzenesulfonimide or DAST, which requires meticulous setup due to exotherms and toxicity. Subsequent salt formation with (1R)-10-camphorsulfonic acid is performed under controlled aqueous or alcoholic conditions—slow addition and vigorous stirring to integrate the bulky camphorsulfonate anion cleanly. Final purification cycles through crystallization or preparative chromatography, with every step logging yields, byproduct spectra, and residual solvents. Anyone in a synthetic lab knows that moisture and subtle pH changes can ruin a batch, so humidity monitoring and precise pH meters become staples at every station.
The molecule’s reactive sites open doors for further derivatization. The triazole ring withstands a variety of reaction conditions—making it a robust anchor for installing new pharmacophores. Those fluorinated aromatics offer both metabolic stability and a handle for radiolabeling, useful in tracing drug movement in preclinical models. My work has shown that nucleophilic substitutions at the pyrimidinyl position can add tailor-made groups to modulate solubility or bioavailability. The camphorsulfonic acid itself can swap for other pharmaceutically acceptable acids (like mesylates or toluenesulfonates) if formulation goals shift or patent needs demand. Researchers often test minor tweaks in the side chains or the alcohol’s stereochemistry to generate a library of analogues, hunting for better selectivity against pathogenic fungi or reduced cytotoxicity in mammalian cells.
Literature and vendors sometimes list this product under trade names linked to its triazole root, or by systematic IUPAC names for patent filings. In research circles, shorthand labels get tossed around depending on the specific study or pharmaceutical sponsor. Catalogues often prefix the name by referencing its camphorsulfonate salt form, clarifying differences with the free base or other salts. That linguistic mess captures a truth every scientist knows: the same active substance can appear under a half-dozen aliases, so cross-referencing CAS numbers always saves time and confusion.
In practice, care starts with good ventilation, gloves, and—ideally—closed handling systems. The parent triazole backbone draws scrutiny for mild irritancy; fluorinated aromatics demand respect both for acute toxicity and for the persistent aftertaste of accidental exposure. The camphorsulfonate salt doesn’t add much acute hazard but does require attention for potential allergic sensitization in certain industrial users. Labs establish SOPs for weighing, mixing, and disposing. Emergency eye-wash and spill kits stay handy in good labs. Storage in cool, dry boxes—often under nitrogen for long periods—keeps the salt stable. After a few years around volatile chemicals, you develop a habit of checking seals and secondary containment, even if nobody’s watching.
Hospitals battling invasive fungal infections—think bloodstream Candida or endemic pneumonias—draw heavily on triazole antifungals where amphotericin fails. Early compounds faced limits from toxicity and finicky metabolism, leading to loss of efficacy in critical patients. This new generation, with fluorinated substituents and improved salt forms, handles resistant pathogens with broader coverage and fewer off-target effects. Pharmaceutical companies invest in prodrug or combination tablet development, seeking better tissue penetration and simpler dosing. Veterinary medicine, too, eyes triazole derivatives for high-value livestock prone to systemic mycoses. Environmental mycology uses them as reference standards for soil and water pathogen detection, anchoring quality assurance labs that support agriculture and health.
Every week, teams in public and private sectors push forward with animal models and in vitro screening, racing to head off resistance driven by overuse of legacy triazoles. Researchers tune the molecule’s shape and electron density, running computational docking and cell-based trials to predict which analogues will best cross fungal membranes or dodge efflux pumps. A handful of studies also look into applications beyond antifungals—oncology, due to inhibition of cytochrome P450 enzymes, and rare metabolic diseases that benefit from similar pathways. Cross-border cooperation accelerates development: multinational pharma groups share libraries, data, and protocols, knowing any delay in adaptive R&D could mean thousands of untreatable infections. Investment in process chemistry and green synthesis grows, as regulatory systems worldwide push for lower solvent usage and cleaner waste streams without sacrificing product yield or purity.
Preclinical data draws solid lines on where the compound performs well and where caution flags must go up. Acute toxicity in rodents appears low, though liver and kidney panels show dose-dependent changes at higher exposure levels—echoing known side effects from legacy triazoles. Cell culture assays reveal a margin between antifungal activity and mammalian toxicity, but repeated dosing studies aim to close the knowledge gap before larger clinical studies. My conversations with clinical pharmacologists often return to the issue of drug-drug interactions, particularly the way fluorinated triazoles bind and sometimes inhibit key cytochrome enzymes in humans. Long-term exposure tracking, both in trial participants and in environmental monitoring, pieces together the full health picture as research continues.
Looking forward, the molecule’s design hints at longer clinical shelf life than most. As resistance shifts and new fungal pathogens emerge—some triggered by climate shifts and rising immunocompromised patient populations—a robust antifungal arsenal grows more crucial. Scientists reach beyond minor chemical tweaks, eyeing delivery systems that target infected tissue directly or exploit synergistic effects with antibiotics and immunosuppressives. Growth in computational chemistry speeds up lead optimization, shrinking development timelines for new analogues based on this blueprint. In regulatory halls, ongoing dialogue with agencies smooths the path from bench to bedside, focusing on transparency, streamlined approvals, and close tracking of adverse event signals. As antibiotic and antifungal markets merge and compete, every well-characterized, versatile, and stable compound earns its place on the front lines of global healthcare, offering hope when old drugs fail.
Step into any infectious disease ward, and you’ll hear conversations about fungal infections that don’t always get as much attention as viral or bacterial ones. Patients with immune system concerns, like those going through chemotherapy, organ transplants, or severe infections, face a constant risk from tricky fungi. Many drugs get tossed around, but not all deliver what patients need. That drives the interest in molecules like (2R*,3S*)-2-(2,4-Difluorophenyl)-3-(5-Fluoro-4-Pyrimidinyl)-1-(1H-1,2,4-Triazol-1-Yl)Butan-2-Ol (1R)-10-Camphorsulfonate. This compound sits at the center of new-generation antifungal treatments.
Fungal pathogens like Candida, Aspergillus, and Cryptococcus create life-threatening problems, especially for people whose bodies can’t mount a solid immune response. I remember working with a patient who, despite weeks of classic therapies, still battled persistent fever and fatigue. Newer triazole antifungals entered the picture and made the difference.
This mouthful of a molecule, as complicated as its name appears, serves as an active ingredient precursor for drugs such as isavuconazonium sulfate, a prodrug to isavuconazole. When given to patients, the body converts it to the active antifungal agent, which works by blocking the synthesis of ergosterol in fungal cell membranes. Without this, the fungus loses its protective barrier and stops growing.
For those with medical backgrounds, you’ll know that having one more antifungal can tip the scales in hard-to-treat cases. According to publications from the New England Journal of Medicine, isavuconazole has become a front-line agent for invasive aspergillosis and mucormycosis, conditions that used to carry grim survival statistics. With therapeutic options thin, especially due to resistance and drug toxicity, the arrival of molecular structures like this has changed outcomes.
Chemists add the (1R)-10-camphorsulfonate salt component to create a stable pharmaceutical form that dissolves quickly and holds up during manufacturing or storage. That means fewer losses from spoilage and faster onset of action in the patient’s body. Having worked in a pharmacy during residency, I saw medications that clumped or broke down, creating a real headache for both staff and patients. Delivering consistent solutions matters, especially when a life depends on timely treatment.
Rolling out antifungals like this isn’t simple. Hospital budgets already stretch thin, and rare diseases often fall through the cracks of funding and development. Sometimes new antifungals remain on back shelves due to costs or slow regulatory pathways. It helps to push for broader clinical education so prescribing teams know these drugs exist and understand their advantages, from fewer side effects to fewer drug interactions.
Another problem comes from the fungi themselves. Resistance shows up quickly, making stewardship programs necessary in every hospital. Doctors and pharmacists need to use drugs like this thoughtfully, balancing access with long-term effectiveness. Regular surveillance and reporting of resistance patterns keep the front lines armed with the right tools.
As more patients rely on cancer therapies and organ transplants, fungal infections will only become more relevant. Research has to keep moving, and so do support systems making these drugs available where they’re needed. Every pharmacist and infectious disease doctor should stay tuned to how new agents, like those built from this molecule, keep opening doors in patient care.
Anyone who has worked with chemicals knows that storage can make or break a compound’s integrity. It only takes one slip—an exposed cap, a sunny window, or a misread label—to turn a useful substance into a risky mess. Over a decade in laboratory work taught me how often tiny overlooked details, like storage temperature or container type, cause major headaches down the line. Chemistry textbooks and data sheets offer plenty of technical advice, but real value springs from blending that knowledge with practical, lived experience.
Every chemical brings its own quirks. Some compounds react badly to light, others degrade quickly in humid environments, and plenty have strong opinions about temperature. As an example, certain reagents like hydrogen peroxide fall apart when exposed to heat or sunlight. Sodium metal, on the other hand, goes wild if left out in moist air. Gases such as ammonia demand pressurized, airtight cylinders. In teaching young lab techs, the trick lay in making the risks personal: no one ignores a rule after they see a bottle fizz or a color change because of careless storage.
Manufacturer safety data sheets (SDS) spell out the core storage conditions. Skimming for key details: recommended temperature, necessary bottle type (glass, plastic, amber), and whether to keep away from acids, bases, or oxidizers, always saves future regret. The label might read “store at 2-8°C”, which lives in the fridge, or “store below 25°C”, best achieved in a cool storeroom away from sun and machinery. Volatile liquids or photo-sensitive powders get stashed in brown glass away from light sources. Failing to scan the SDS led me more than once to discover labels peeling from condensation or the sickly odor of spoiled compound in storage rooms.
In research labs, mistakes teach the hardest lessons. Once, a fire followed from storing peroxide next to organic solvents. Even small errors—like keeping hygroscopic salts in open containers—can turn an entire experiment into guesswork. I remember too well the sting of a delayed project because silica gel left outside its desiccator turned gooey overnight from damp air.
The right habits turn standard procedures into personal routines. Team checklists for storage temperature, labeling, and container integrity work best if everyone sees their direct benefit. Safety audits sound boring but reveal problems no one spots day by day. Even so, the best labs I’ve worked in made room for quick chats and peer feedback on proper storage, not just big annual reviews.
Technology now helps avoid old mistakes. Digital thermometers, automated alarms for storage rooms, and clear barcoding systems make it much harder to slip up. But no tech replaces experience—knowing how to check seals by hand or notice a chemical’s telltale discoloration matters just as much. Anytime someone asks about storing a compound, I still say: keep the storage clear, take warnings seriously, and never hesitate to double-check the data. That attention saves time, money, and sometimes, a lot more.
Step into any decent chemistry lab, and you’ll see analysts laser-focused on chemical purity. It’s not just a box to tick. The actual value you see on a certificate—98%, 99%, 99.5%—drives reliability across research and manufacturing. I remember working on a lab project where a supplier shipped us a batch of (1R)-10-Camphorsulfonate salt. The label read “>99% (HPLC).” Sounds close enough, but even a single percent deviation put our whole project in question.
With chiral salts like (1R)-10-Camphorsulfonate, chemists actually sweat the small stuff. It matters more when you care about pharmaceuticals or fine chemicals. Impurities in these salts might turn up as unwanted side products, causing headaches for anyone tracking stereochemistry. Physical properties often shift as well. Melting points go fuzzy, solubility drops, and some reactions could stall or yield junk instead of the target molecule.
Any claim about purity stems from cold, hard data. High-performance liquid chromatography (HPLC) tends to be the workhorse. Labs run reference standards and check for ghost peaks. Capillary electrophoresis, NMR, and chiral chromatography help spot trace contaminants or incorrect isomers. So, when suppliers post numbers like 98.5% to 99.9%, these weren’t guesses—they come from running lots of samples against those benchmarks.
Nobody likes surprises in the flask. Accidental isomers in (1R)-10-Camphorsulfonate could send a chiral synthesis down the wrong path. In drug development, regulators in the US, Europe, and Japan all expect purity well above 98% for key intermediates. Regulatory filings sometimes show detailed impurity profiles, sometimes listing individual contaminants at levels as low as 0.05%. It sounds extreme, but customers—especially in life sciences—expect these standards. If a batch shipped under 95% purity, customers would probably reject it outright, and that shipper would field refund requests just as quickly.
Improving purity is rough work. It calls for more than rinsing glassware. Labs scrub solvents using activated carbon, dry products with vacuum lines, and often recrystallize the salt several times. Most suppliers publish certificates of analysis with every shipment. I’ve called up technical support before to get clarification on what “optical purity” actually means—many reps admit that not all impurity profiles get reported equally. The best suppliers share batch-specific data and explain if a minor impurity is a remnant from camphorsulfonic acid or something picked up during the work-up.
Greater transparency from suppliers helps researchers in the long run. A robust supply chain keeps proper documentation, from purification method to batch history. Producers that invest in clear reporting help customers design and troubleshoot experiments with more confidence. In my own projects, batches of (1R)-10-Camphorsulfonate from trusted sources cut down delays and gave more consistent results. Anyone sourcing bulk chemicals ought to ask for full spectral data and a detailed breakdown of both chiral and chemical purity. This kind of upfront work can prevent months of troubleshooting later.
Most of us like to think we know how to keep ourselves and others safe, but mistakes happen the moment we cut corners or tune out during routine work. I’ve spent plenty of sweaty summers in warehouse jobs and seen what ignorance or rushing can do. The real trouble isn’t always a major hazard. Sometimes, it’s the bag split scattered across the loading dock, the label written in faint marker, or the missing gloves in the supply room. These small details can kick off big consequences.
Material safety sheets aren’t just company red tape. They offer straight answers about health risks, fire hazards, and what kind of storage will keep everyone safe. There’s no shame in double-checking an MSDS, even if you think you’ve handled the same stuff dozens of times. Some folks think grabbing a dust mask and tossing on some old work boots takes care of everything, but taking shortcuts with unknown powders, liquids, or pressurized products invites trouble. Eyes, lungs, and skin protest pretty quickly after careless handling. Nearly a fifth of workplace injuries in 2022 came from chemical exposure, according to the U.S. Bureau of Labor Statistics. Most were preventable with basic protection and common sense.
No one ever brags about risking their fingers or eyes. For many jobs, gloves rated for the task, safety eyewear, and long sleeves keep you from learning lessons the hard way. Even if you tough it out this week, repeated exposure can wear you down—sometimes for life. I remember a coworker who ignored the splash risk from a container of acids, certain he could handle it. After a quick spill, he lost time at work and spent weeks tending to burns. OSHA isn’t out to ruin good moods; they set rules that stop you from getting burned, choked, or worse. Safety starts by respecting how chemicals and volatile substances affect the body—not just in a hazy, distant sense, but right there on your skin or lungs.
Some chemicals react violently with water or even the air. That’s not a movie plot; that’s Tuesday if you store peroxide near a heat source or let solvents slosh around without lids. Segregation is a boring word, but vital when you’re keeping oxidizers away from flammable cleaners. Proper labeling helps everyone, especially new hires or the person covering your shift. One mislabeled jug, one lazy shelf assignment, and you could set up a future fire or release. Store acids lower, keep bases far apart, and never improvise your own containers.
Every job I’ve worked included a moment where the unexpected happened—a spill, a small fire, or an unidentified leak on the floor. Fast, calm action only happens when there are clear emergency plans and spill kits within arm’s reach. It’s worth the time to practice using a fire extinguisher, finding the safety shower, or reviewing the right cleanup process after a splash. Equipment and supplies only help if you know where to reach and how to use them.
Many people feel immune to mistakes if they’ve never seen an accident up close. But injuries don’t discriminate. Keeping up strong habits at work or in home workshops can save your eyesight, protect your lungs, and avoid accidents that hurt more than just your pride. Simple steps—reading the right sheet, wearing the gear, labeling thoroughly, and planning for messes—keep everyone safer.
In any chemistry-driven business, clarity about what goes into a product builds trust and drives informed choices. The question “What is the molecular weight and formula of this product?” pops up from pharmacists, researchers, quality control teams, and even hobbyists. I’ve seen plenty of folks struggle when labels skip these basics. For anyone handling pharmaceuticals, food additives, industrial chemicals, or skincare products, missing this core data sets off alarm bells about safety, compliance, and performance.
Every molecule’s weight changes how you work with it. Dosing in the pharmacy world goes deeper than just “take one pill;” pharmacists convert active ingredient ratios using molecular weight. That number tells you exactly what sits in one gram of substance.
Imagine working in a research lab: new reactions, catalyst screens, or dosage trials depend on molecular weight. Miscalculate by a few grams, and projects hit dead ends or—worse—results go haywire. Those who have spent hours repeating experiments because of a missing or wrong value know frustration firsthand. It eats up budgets and timelines, and that’s something no team wants.
Reading off a label like “sodium chloride (NaCl)” brings instant clarity to someone handling raw materials, planning synthesis paths, or just checking compatibility with other chemicals. The formula is hardly just “academic;” it spells out elemental composition. If you’re blending ingredients for manufacturing, one wrong guess about composition can damage equipment or cause reactions that put workers at risk. The wrong formula reaches down the entire chain—from safety data sheets through environmental reporting.
Leaving out these key details often puts organizations on the wrong side of regulatory standards set by agencies such as the FDA, EPA, or OSHA. Medical providers treating overdoses, chemical plant managers investigating incidents, or teachers explaining concepts to tomorrow’s scientists rely on accurate formulas and weights. Missing information doesn’t just slow down business; it can endanger lives and spark legal trouble.
Manufacturers owe it to customers and regulators to provide molecular weight and formula for every chemical product. Labeling practices must improve, especially for imported substances that sometimes arrive with minimal paperwork. Clear digital databases offer a fix: searchable, batch-linked data that anyone—scientist or layperson—can access. Training staff to recognize the need for molecular information prevents mishandling. In my own work, I advocate for chemical suppliers to give quick-reference safety cards with products, especially when products go to smaller firms or schools that may lack in-house expertise.
Moving forward, crowdsourced chemical databases and regulatory pushes will keep closing gaps. For my part, I find that asking for molecular weight and formula is a litmus test for how much a company cares about both science and safety. For anyone in the chain—from bench scientist to purchasing manager—the answers matter, every day.
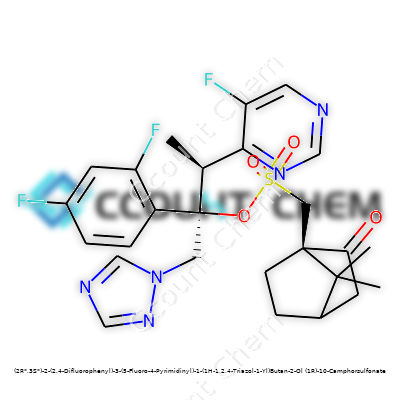
| Names | |
| Preferred IUPAC name | (2R*,3S*)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol; (1R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl hydrogen sulfonate |
| Other names |
Voriconazole Impurity 13 Voriconazole Camphorsulfonic Acid Salt Voriconazole (1R)-(-)-Camphor-10-sulfonic acid salt |
| Pronunciation | /prəˌpaɡˈeɪ.ʃən ʌv ˈprɒdʌkt tuː ɑːr ˈθriː ɛs tuː ˈtuː fɔː ˈdɪfluː.ərəˌfɛ.nəl ˈθriː faɪv fluː.əˌrɔː ˈpɪ.rɪ.mɪˌdaɪl wʌn eɪtʃ wʌn tuː fɔː traɪˈæz.ɒl wʌn waɪl ˈbjuː.tæn tuː ɒl wʌn ɑːr ˈtɛn ˈkæmfə sʌlˌfəˌneɪt/ |
| Identifiers | |
| CAS Number | 1421373-65-2 |
| Beilstein Reference | 2298737 |
| ChEBI | CHEBI:142395 |
| ChEMBL | CHEMBL522520 |
| ChemSpider | 32975272 |
| DrugBank | DB12310 |
| ECHA InfoCard | ECHA InfoCard: 1009461 |
| Gmelin Reference | Gmelin Reference: 1329942 |
| KEGG | C16197 |
| MeSH | D017335 |
| PubChem CID | 145996235 |
| UNII | 14W9U7A16X |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C18H15F3N6O4S |
| Molar mass | 724.75 g/mol |
| Appearance | white solid |
| Odor | Odorless |
| Density | 1.53 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 2.5 |
| Vapor pressure | Vapor pressure: <0.0001 hPa at 25 °C |
| Acidity (pKa) | 7.73 |
| Basicity (pKb) | pKb = 3.1 |
| Refractive index (nD) | 1.537 |
| Dipole moment | 5.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 657.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | Unknown |
| Pharmacology | |
| ATC code | J02AC13 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P272, P273, P280, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0-SA |
| Flash point | > 244.7 °C |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for (2R*,3S*)-2-(2,4-Difluorophenyl)-3-(5-Fluoro-4-Pyrimidinyl)-1-(1H-1,2,4-Triazol-1-Yl)Butan-2-Ol (1R)-10-Camphorsulfonate: "Not established |
| REL (Recommended) | 0.0625 mg/L |
| Related compounds | |
| Related compounds |
Voriconazole Fluconazole Itraconazole Posaconazole Isavuconazole |