Chemical progress usually follows surprising turns, and 2-toluenesulfonic acid has left its footprint across decades. I remember poring over old chemical journals, tracing names like Friedrich Beilstein, and seeing how the late 19th century laid the groundwork for strong organic acids. Early chemists wanted acids stronger than carboxylic ones, but that still brought organic solubility and reactivity. The discovery of toluenesulfonic acids offered this breakthrough. The methyl group on toluene cranks up reactivity, making these compounds mainstays for organic synthesis and turning simple aromatic substrates into true powerhouses. Industry grabbed the compound, manufacturers scaled up in bulk to match the growing needs in dyes, drugs, and later, plastics. Over generations, the original preparation and purification methods were tweaked, but the same basic backbone held steady, showing the rugged and reliable nature that today's chemists still value.
2-Toluenesulfonic acid appears most commonly as white, needle-shaped crystals, easy to recognize and handle in the lab. Structurally, it features a methyl group at the ortho position to the sulfonic acid, balancing reactivity with reasonable stability during storage and handling. Chemists expect certain behaviors: strong acidity, good solubility in polar solvents, and solid reliability as a catalyst or intermediate. In my experience, when a synthesis needed a robust acid that stood up to the workhorse tasks where sulfuric acid faltered, 2-toluenesulfonic acid often found its place on the shelf, ready to take over jobs like esterification, polymerization, or resin production. Few compounds are as trusted across academic, research, and manufacturing setups.
2-Toluenesulfonic acid has a sharp melting point, usually crystallizing around 105°C, and offers high thermal stability, which lets it play a role in high-temperature reactions safely. Its solubility in water is substantial due to the strong sulfonic acid group, yet the benzene ring and methyl group maintain good solubility in some organic solvents like ethanol or acetone. The compound itself stands out for its non-volatile, hygroscopic nature. Anyone who's spilled a flask can tell you: leave it out, and it absorbs moisture quickly. On the reactivity front, the acid group is strong enough to protonate most organics that need activation, yet rarely reacts too aggressively with typical functional groups—an essential balance for many syntheses.
Producers sell 2-toluenesulfonic acid in grades tailored to chemistry, pharmaceuticals, or industrial catalysis. Bottles carry the CAS number 88-20-0, clear purity markers, and statements regarding water content since the hydrated form can affect reaction stoichiometry. Product labeling usually highlights whether the compound comes in monohydrate or anhydrous form. Details like the presence of iron, chlorides, or any coloring matter remain vital to industrial buyers, since minor contaminants sometimes throw off sensitive polymer or drug synthesis. Specifications often insist on colorless crystals and minimal residue on ignition, supporting use in high-purity end products.
The classic route to 2-toluenesulfonic acid starts with direct sulfonation of toluene using concentrated or fuming sulfuric acid under carefully controlled conditions. Temperature and proportion keep ortho-substitution favored, sidestepping production of the para isomer, which dominates under less controlled setups. The process gears up for industrial batch scales easily, and yields stay high when reaction conditions are monitored carefully. In my time running similar reactions, patience with extraction and diligent crystallization have always paid off. Modern chemical engineering improved product isolation, drying, and by-product management, but the roots of the process have barely changed for generations.
In synthetic chemistry, 2-toluenesulfonic acid gives, and it receives. Its acid group welcomes the formation of toluenesulfonates, which act as handy leaving groups in displacement reactions. They're crucial in producing a range of chemicals from fine drugs to fragrances. This acid also activates alcohols for substitution, making esterification smoother and faster compared to weaker acids. Chemists often make sulfonate esters that help step up organic transformations or aid in protecting reactive sites. Sugars, peptides, and complex small molecules get assembled on its backbone, and it’s a staple in peptide coupling and protecting group installation. Treating it with phosphorous reagents, one can tweak its properties or couple it onto more exotic frameworks for specialty industrial tasks.
Across catalogs, 2-toluenesulfonic acid pops up under names like o-toluenesulfonic acid, ortho-toluene sulfonic acid, and simply OTS-acid. Commercial products reference its three-letter abbreviation, but also list its full IUPAC name for clarity. International shipments sometimes label it “o-Toluenesulfuric acid,” especially outside the English-speaking market, emphasizing the need for vigilance during global trading and cross-reference of technical sheets to avoid confusion with its para-isomer.
Dealing with 2-toluenesulfonic acid in the lab or plant environment calls for careful PPE and constant eye on safety. The crystals and dust can irritate eyes, skin, and the respiratory tract; even short exposures lead to redness and discomfort. Spills call for swift cleanup since the acid picks up water fast and can corrode metal benches or equipment. Over the years, regulations have called for sealed containment, fume hoods, splash-resistant gloves, and rigorous labeling of containers. In larger facilities, SOPs require regular training and record-keeping that tracks storage and disposal—there’s no room for shortcuts. Waste gets treated with neutralizers like base or carbonate, then tested before release.
Industry and research lean heavily on 2-toluenesulfonic acid for its unique blend of strength and selectivity. In organic synthesis, it shines as a catalyst for ester and ether formation, where cleaner products outweigh minor costs. Resin manufacturers count on it for heat-cured polymers — like phenol formaldehyde or epoxy systems — boosting network strength and chemical resistance. Formulators in the pharmaceutical world pick it as a counterion to form active pharmaceutical ingredient (API) salts, tuning solubility and bioavailability in finished drugs. Water treatment, synthetic dye production, and oil refining operations also rely on its reactivity profile, proving its usefulness in surprising corners of the chemical world.
Every year, journals publish fresh studies into sulfonic acids, but 2-toluenesulfonic acid maintains a steady stream of interest for both academic and commercial R&D. Researchers test new catalytic cycles for greener processes by using this acid as a less hazardous replacement for mineral acids, trying to cut risk and environmental costs by sidestepping harsh reagents. Peptide chemists keep refining ways to use it in coupling and deprotection, reducing waste and increasing yields for complex drug molecules. New derivatives or solid-supported versions enable reuse in continuous-flow reactors, trimming costs for bulk production. Scientists exploring advanced materials—such as proton exchange membranes or eco-friendly plasticizers—often rely on the robust acidity and stability that 2-toluenesulfonic acid offers.
Toxicology work so far flags eye and skin irritation as the main hazard, but animal studies and workplace investigations watch for longer-term effects. Acute toxicity numbers stay relatively low, and accidental ingestion or inhalation rarely proves fatal at the concentrations used in industry. Chronic studies don’t show evidence of carcinogenicity when used responsibly, but the compound’s acidity and reactivity mean that careless handling can cause ulceration or longer-term mucous membrane problems. Regulatory bodies like OSHA and ECHA keep exposure limits conservative, responding to new data as it comes in and making sure workplaces apply protective measures. Disposal studies watch for possible water contamination, as even trace amounts can drop pH and harm aquatic life.
2-Toluenesulfonic acid might seem old-school, but the future offers more than basic catalysis or intermediate status. As green chemistry advances, this acid serves as a bridge between mineral and organic acids, opening up scalable processes for biodegradable plastics, fine pharmaceuticals, and safer consumer goods. Continued work on solid-supported or recyclable versions could mean less waste and lower production costs. New research targets its use in flow reactors and hybrid organic-inorganic catalysis, giving it a renewed role in sustainable chemistry. Anyone following polymer science or pharmaceuticals should expect to see more about 2-toluenesulfonic acid surfacing in innovation pipelines and next-gen chemical engineering projects, building on the long tradition that brought this simple aromatic acid from the early days of chemistry into the modern age.
2-Toluenesulfonic acid, often just called TsOH, plays a big role in many labs and factories. Its structure, based on toluene with a strong sulfonic acid group, makes it a heavy hitter among acids. It’s stronger than acetic acid but easier and safer to handle than sulfuric acid. Chemists favor it as a catalyst for sticking molecules together, breaking them apart, or starting up polymer chains. I remember tackling an undergraduate esterification lab after struggling with poor yields year after year. Someone swapped in TsOH for our usual acid. The difference? We got product in hours instead of overnight. That kind of reliability explains why researchers keep bags of crystalline TsOH close at hand.
On the industrial side, businesses look for acids that do the job without tearing up equipment or creating headaches with byproducts. TsOH stands out here. In pharmaceuticals, it helps build active ingredients and their intermediates. Its solid form means you can weigh it out, toss it into a reaction, and get a predictable result. For example, companies making antibiotics use TsOH to guide crucial steps where getting the wrong product—just a few percent—means wasted time and expense. Its acid strength gives precise control, and cleanup gets easier, since it stays separate from many solvents. Fewer surprises, fewer safety reports.
Beyond drugs, TsOH shapes the look and feel of everyday products. Resin shops use it in creating paints and varnishes. The acid sets off polymerization, driving small molecules to form long chains. That cuts down paint curing time, boosts gloss, and sharpens color resilience. Textile manufacturers bathe fabrics in dyes fixed into place using TsOH. It gives rich blues, reds, and blacks that last through wash after wash. Even electronics benefit—some circuit board makers etch copper with help from TsOH mixtures, carving precise lines for today’s compact devices.
With all this utility, there’s still responsibility tied in. TsOH eats away at skin and mucous membranes. Splashes demand prompt rinsing and proper gloves, goggles, and lab coats go on before the bottle opens. Disposal counts, too. Dumping sulfonic acids can harm water sources and wildlife. Industry regulations rightly require neutralization before waste heads down the drain. Over the years, companies have installed scrubbers to catch fumes and systems to neutralize leftover acid, but accidents still happen without training and attention. Strict safety routines protect workers and keep pollution in check.
Teams can boost TsOH’s utility and reduce danger. Automation helps by measuring and mixing the acid safely. Greener manufacturing relies on closed systems to keep acid traces away from staff and the environment. Recycling solvents and recovering TsOH from finished reactions lowers costs and keeps work sites cleaner. I’ve seen some clever solutions in university labs—using nontoxic buffers to mop up acid after reactions instead of strong alkalis that just shift the hazard somewhere else.
TsOH will probably remain a staple for chemists, engineers, and product designers. Its versatility, reliability, and proven performance mean that attention now shifts more and more to safe handling and creative new applications.
2-Toluenesulfonic acid shows up in labs and factories everywhere you look. It’s a strong organic acid often used to help speed up chemical reactions, clean equipment, and manufacture dyes, medicines, and polymers. The problem doesn’t hide in the name or its everyday uses. The danger builds up quietly if you don’t pay attention to how you store, use, or dispose of it.
My time in an industrial setting showed me what happens when workers underestimate compounds like this. 2-Toluenesulfonic acid isn’t something you want on your skin, let alone in your eyes or lungs. Even a splash causes nasty burns and irritation. Vapors can trigger coughing and throat pain. Gloves, goggles, and face shields never felt optional; anyone skipping them often learned the hard way.
Spills create even bigger headaches. The acid reacts with water and many metals, producing heat and sometimes flammable hydrogen gas. That can spiral if cleanup doesn’t happen fast and with the right neutralizers. Fire departments urge every facility to prepare for this exact situation.
Short-term pain isn’t the end of it. Breathing in the dust can damage the nose and lungs—think chronic sore throats, coughing, and nosebleeds for anyone exposed regularly. The acid stings the skin and eyes, but repeated contact leads to cracked skin, blisters, and permanent scarring. Even if someone gets lucky with a one-time encounter, co-workers and health officials remind us that chronic exposure is way more common than a single dramatic accident.
Dumping it carelessly isn’t just bad for people—it damages local water, soil, and wildlife. If the acid leaks into drains or streams, it changes the pH and stacks up toxins for fish and plants. Factories caught polluting risk big fines, but more importantly, entire ecosystems can take years to recover. Keeping acids sealed, labeled, and contained always made cleanup drills worth the effort in my workplace.
So what do you do when you can’t avoid using it? Detailed training makes a difference. I remember older coworkers drilling into us: Always check the Material Safety Data Sheet. Never eat or smoke where acids get handled. Clean spills immediately, don’t leave contaminated rags lying around, and fix ventilation if even a faint odor shows up. Double gloves and chemical-resistant aprons slow you down, but they stop trips to the emergency room.
Some companies now look for greener acids or solid forms that are less likely to spill. Enclosed systems and better fume hoods also tackle most of the airborne risks. Emergency wash stations and team drills mean you’re not alone if something goes wrong.
The lesson sticks with anyone who’s watched a coworker run for the eyewash. 2-Toluenesulfonic acid can power big advances in science and industry, but every shortcut taken with safety has led to avoidable injury or harm to the environment. Keeping it out of water and off skin isn’t just regulation; it’s common sense. People value their health, and those who treat chemicals with respect work smarter, not just faster.
2-Toluenesulfonic acid, or TsOH, is one of those key substances that keeps showing up in labs and manufacturing plants around the world. The formula for this compound is C7H8O3S. To break this down: you get seven carbon atoms, eight hydrogen atoms, three oxygen atoms, and a single sulfur atom—packed together in a way that makes TsOH a tough, reliable acid for many kinds of chemical reactions.
It’s not just an academic exercise to memorize that formula. If you’re working in pharmaceuticals or fine chemicals, this piece of information steers safe storage, handling, and application. Once, in a project preparing an active pharmaceutical ingredient, the proper calculation of reagents came down to grasping each atom in the formula. A misstep here means poor yields or, worse, safety risks.
Too often, folks overlook the sulfur atom in the formula. Skipping it even once in stoichiometry calculations can lead to under- or over-acidification during production. TsOH holds its ground as a strong acid because its sulfonic group pulls electron density, giving that hydrogen plenty of willingness to break loose in solution. All of this traces right back to how the atoms line up, and the only way to keep it straight is to recall the true formula: C7H8O3S.
You don’t need to wander far in the chemical industry to see TsOH at work. It’s used to catalyze reactions in organic synthesis—helping to build bonds quickly in a controlled environment. Its power comes from the sulfonic acid group, which drives strong acid behavior, outpacing many mineral acids in certain applications. From running Fischer esterification to prepping polymers, keeping the right formula in mind guides how much TsOH goes in and what byproducts might form.
Knowing exactly what the atoms are means knowing how TsOH behaves. Those three oxygen atoms and sulfur flag TsOH as corrosive and potentially harmful with skin or eye contact. Proper knowledge of its formula connects to understanding Material Safety Data Sheets and chemical hazard symbols in the workplace. My own experience dealing with accidental skin exposure reinforced the need for precise safety training based on clear chemical identification, not guesswork.
Gaps in understanding basic chemical formulas create problems not only for students but for professional lab workers, too. Even with modern apps and calculators, solid chemical literacy builds trust in calculations and keeps projects on track. The answer to “What is the chemical formula of 2-Toluenesulfonic acid?” matters anytime chemicals get measured, stored, or transported.
Promoting training sessions and regular review of chemical labeling go a long way to avoid costly mistakes. It also sharpens confidence for those entering the industry. People new to laboratory work often need extra review—not just on handling but on the basics of formulas and the structure of what they’re using daily.
Knowing the formula C7H8O3S opens doors in synthesis, process safety, and chemical education. This single tidbit saves time, supports quality control, and keeps labs running smoothly. Miss it, and the risks multiply—not just for products but for people.
Many people encounter chemicals in their line of work, sometimes without fully understanding the risks. 2-Toluenesulfonic acid might not be as talked about as the acids you remember from chemistry class, but don’t let that make it seem harmless. It draws immediate respect from anyone who’s accidentally spilled it or watched a container sweat in humidity. That bite of acrid vapor in the nose tells you—this chemical likes to draw water and pack a punch.
Some labs and factories keep their 2-Toluenesulfonic acid in bulk, sometimes stacked awkwardly in storerooms or shoved behind something else to “keep it out of the way.” On a sweaty summer afternoon, you walk in and see white, clumpy crust around the drums. That’s not just poor housekeeping—that’s a recipe for trouble. The chemical pulls moisture out of the air, and with it come corrosion and clogs. If you’ve ever struggled with jammed dispensing pumps or spent hours cleaning up sticky residue, you know how big a pain that turns into.
I spent time working in a manufacturing site where storage protocols sometimes got ignored in favor of speed. One mistake—broken packaging left open overnight—meant dealing with hardened, stuck-together powder by morning. It halted production for half a shift, cost hundreds in wasted material, and put two people in the clinic with skin rashes. People remember those days. Over the years I realized that good science relies as much on clean storage practices as it does on clean chemistry.
You don’t need to break the bank or build a bunker to store 2-Toluenesulfonic acid safely. Start with a good, tight container. Most folks I know use polyethylene or glass. These materials stand up to strong acids, hold tight against moisture, and don’t rust. Leaving the bag or can open between uses just swells costs and worries. If the lid fits snug, you keep out the damp air, which means the acid doesn’t cake or react with other stuff in the storeroom.
Dry, cool storage always wins the day. A shelf that stays below 25°C keeps the chemical from breaking down or turning into a sticky, tricky mess. Sunlight might look harmless pouring through a window, but direct rays heat up containers. The acid can change color, thicken, or even damage the packaging. Lighting things well helps, but ultraviolet rays should never hit the product directly.
If you want to keep your workplace out of trouble, take basic rules seriously: Label every container clearly. No scribbling product names in marker, hoping people guess right. Regular checks of seals and packaging show problems before they grow. If someone finds corrosion or damage, they tell a supervisor the same day. Slipping on safety here brings big headaches later—damaged containers leak, and leaks lead to lost time and injuries.
Personal experience taught me that the safest shop runs on routines and respect for the tools and products you work with. Following storage guidelines turns what might seem a tedious chore into a guarantee of an uninterrupted workweek. It protects the bottom line and, more importantly, the people in the building. 2-Toluenesulfonic acid doesn’t forgive shortcuts, and neither should anyone who handles it.
2-Toluenesulfonic acid isn’t something most people keep in a cupboard at home. It's a powerful sulfonic acid, often used in labs and chemical production for its effective catalytic properties. Having spent some time in chemical labs, I’ve seen professionals respect this compound for good reason. It can cause chemical burns, severe irritation on contact, and harmful effects if inhaled or swallowed. The facts are simple: accidents usually come from rushing, ignoring protocols, or working without enough protection.
Gloves, goggles, and a lab coat are basic. Thick nitrile or neoprene gloves stop splashes from reaching skin. Regular latex gloves just don’t stand up to strong acids. Safety goggles with side shields keep sprays away from eyes—something many forget until it’s too late. Cover skin with lab coats or chemical-resistant aprons. The acid eats through thin fabric quickly, so heavier protection wins every time. Inhalation risks call for respirators, not just open windows.
People working with 2-toluenesulfonic acid often try to cut corners when it comes to air quality. But this isn’t a faint vinegar smell we're talking about. Even at low exposure, the dust or mist can cause throat and lung irritation. Lab work involving acids should always take place in a fume hood. Fume hoods suck harmful gases out of the breathing zone, keeping exposure down.
Never store this acid anywhere near bases, water-reactive chemicals, or oxidizers. Corrosive cabinets, labeled clearly, protect everyone. Containers must be well-sealed, as the acid absorbs moisture from the air and starts to degrade. I’ve seen careers set back—and facilities locked down—after a mislabeled bottle caused confusion. Keep only the amount you plan to use, and always write the opening date and the handler's name on the container.
No matter how careful people are, spills happen. Know where the nearest eyewash station and emergency shower sit, and make sure they work. A full spill kit nearby (with neutralizers, absorbent pads, and disposal bags) is as valuable as any piece of high-tech lab equipment. Don’t try to clean up a big spill alone—ask for help before someone pays the price in burns or respiratory trouble. Wearing protective gear during cleanup is just as important as during routine handling.
Many problems disappear when training becomes routine, not just a box-ticking chore. Workers who know exactly what to do in a flash, avoid panic. Regular safety briefings make sure nobody forgets the basics, even when things get busy. Watch each other for mistakes—a culture of open communication about errors builds trust and helps everyone keep their guard up.
Disposing of 2-toluenesulfonic acid needs planning, not improvisation. This isn’t the sort of chemical you flush. Collect all waste in a clearly labeled, compatible container, and arrange for pickup by a team that’s trained for dangerous waste. Anything less risks environmental harm and legal trouble. Keeping records of disposal keeps you, and the environment, out of trouble.
| Names | |
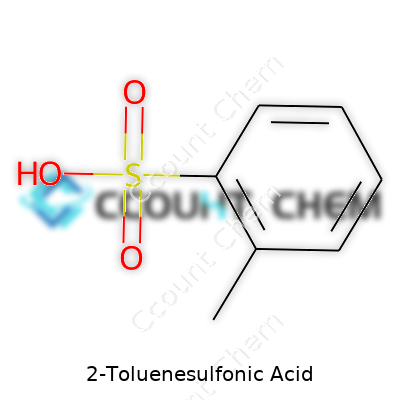
| Preferred IUPAC name | 4-methylbenzenesulfonic acid |
| Other names |
ortho-Toluenesulfonic acid o-Toluenesulfonic acid 2-Methylbenzenesulfonic acid o-Toluolsulfonsäure |
| Pronunciation | /tuːˌluːinˈsʌlfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-20-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Toluenesulfonic Acid**: ``` CC1=CC=CC=C1S(=O)(=O)O ``` |
| Beilstein Reference | 1207935 |
| ChEBI | CHEBI:28766 |
| ChEMBL | CHEMBL608 |
| ChemSpider | 13207 |
| DrugBank | DB04452 |
| ECHA InfoCard | 100.003.292 |
| EC Number | '204-482-5' |
| Gmelin Reference | 82255 |
| KEGG | C01382 |
| MeSH | D014033 |
| PubChem CID | 6077 |
| RTECS number | XS7350000 |
| UNII | O40UQP6WCF |
| UN number | 2587 |
| Properties | |
| Chemical formula | C7H8O3S |
| Molar mass | 190.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.24 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.0 |
| Vapor pressure | <1 mmHg (20 °C) |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | -6.50 |
| Magnetic susceptibility (χ) | -5.3×10^-6 cm³/mol |
| Refractive index (nD) | 1.587 |
| Viscosity | 25 mPa·s (20 °C) |
| Dipole moment | 3.22 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 215.0 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -771.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1621.6 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, causes serious eye damage. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | Hazard statements: Causes severe skin burns and eye damage. Harmful if swallowed. |
| Precautionary statements | P264, P280, P301+P330+P331, P305+P351+P338, P310, P303+P361+P353, P363 |
| NFPA 704 (fire diamond) | 3-0-2-A |
| Flash point | 79 °C |
| Autoignition temperature | 480 °C |
| Lethal dose or concentration | LD50 (oral, rat): 2470 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2470 mg/kg |
| NIOSH | WSH3675000 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | 40 mg/m3 |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid 4-Toluenesulfonic acid Methanesulfonic acid p-Toluenesulfonyl chloride Sulfanilic acid |