Looking back through the annals of organic chemistry, 2-Oxobornane-10-sulphonic acid emerges from a long chain of inventive steps that trace to mid-20th-century studies in camphor derivatives and sulfonic acid chemistry. Early researchers hunting for new reactivity in the camphor framework moved from simple oxidation to more involved substitutions, eventually reaching the territory of sulfonation. Their efforts, fueled by a desire to create versatile building blocks for both industrial and academic uses, led to the first reports of 2-Oxobornane-10-sulphonic acid. The story picks up as applications in dye intermediate synthesis, surfactant development, and specialty catalysis established the molecule’s value. No single institute owns the path—findings from Russian, German, and American labs contribute through scattered journal articles, patents, and conference papers, all pointing to a recurring theme: this molecule offered more than just a chemical curiosity.
Think of 2-Oxobornane-10-sulphonic acid as a hybrid, shaped by camphor’s rigidity and the forceful presence of a sulfonic acid group. Commercial preparations often arrive as free acids, crystalline solids, or in some cases, sodium or potassium salts. Companies involved in fine chemistry and research—Sigma-Aldrich, TCI, Alfa Aesar—have supplied this compound in research and semi-bulk formats. Whether purchased or synthesized in the lab, its unique molecular skeleton gives it a distinctive edge among related cyclic sulfonic acids. Batch consistency counts; I’ve always noticed how purity levels above 98% make all the difference in downstream transformations—impure batches lead to headaches in reaction yields, especially during nitration or reduction steps.
2-Oxobornane-10-sulphonic acid stands out as a white to off-white powder, packed tightly because of its compact tricyclic camphor-based structure. Its melting point hovers above 200°C, and it handles moderate humidity without rapid deliquescence, a blessing for bench chemists. Packed with both a strongly acidic sulfonic group and a rigid ketone at the 2-position, this molecule shows high water solubility, especially in its salt forms, and also tolerates mixing in polar aprotic solvents during reaction workups. The chemical stability holds up well up to 120°C, after which sulfonic groups risk slow decomposition—a fact that always mattered when scaling up dehydrations or heat-driven reactions. Odor remains mild, unlike some other sulfur-containing compounds.
With common lots sourced from suppliers, technical documents specify content above 97% for research purposes, with moisture typically under 1%—low enough to avoid caking. Salted versions (sodium or potassium) carry similar guarantees with minor deviations in pH for aqueous solutions; free acids hover at pH 1-2 in water. Labels often include UN numbers for transport (if classified corrosive) and point out the need for tightly sealed containers. My own experience confirmed suppliers who invest in detailed CoAs and batch-level certificates usually ensure fewer regulatory concerns. Storage away from oxidizers preserves not just the shelf life but also sample purity.
A practical synthesis routes through camphor or camphorquinone, commonly starting with sulfonation using oleum or chlorosulfonic acid under controlled low-temperature conditions. The sulfonation of camphor yields a mix of regioisomers, but the 10-sulphonic acid position dominates after careful temperature and stoichiometry adjustments. Oxidation at the bridgehead generates the ketone, either before or after sulfonation, depending on starting material. For those running small-scale batches, the exothermic nature of sulfonation can't be underestimated—ice baths are mandatory, as our group discovered after one too many instances of runaway reactions. Workups often rely on aqueous extraction followed by acidification or neutralization, with final purification through repeated crystallization or precipitation from ethanol or acetone.
Chemists turn to this acid for electrophilic substitutions, sulfonation cascades, and oxidation-reduction maneuvering. The robust acidic group withstands a variety of transformations: you can esterify it under Fischer or Steglich conditions, convert it to its sulfonate ester for nucleophilic substitutions, or reduce the ketone with common hydride donors to reach various hydroxylated derivatives. Its rigid skeleton resists simple ring opening, so most modifications focus on the periphery—halogenation, nitration, or diazotization can be done on the camphor scaffold itself with tailored reactions. For multi-step synthesis, the acid group often serves as a protecting handle, and I learned early on just how effective its sodium salt form is in aqueous-phase cross-coupling reactions.
Literature pulls together a hodgepodge of synonyms: Camphor-10-sulfonic acid; 10-Sulfo-2-oxobornane; 10-Sulfocamphor; 10-Camphorsulfonic acid; and even 10-Sulfo-2-ketobornane. Common abbreviations in academic texts include CBS acid, BSA (bornane sulfonic acid), and sometimes camphor sulfonic acid, although care is needed, as camphor sulfonic acid can refer to the isomer with no ketone group. CAS number 3144-16-9 typically clarifies any ambiguity in procurement and regulatory paperwork.
Handling 2-Oxobornane-10-sulphonic acid does not compare to working with concentrated sulfuric acid, but skin and eye irritation risks command attention. Even seasoned chemists sometimes learned this the hard way—splashes can sting, and airborne dust occasionally irritates nasal passages. Best practice sets gloves, goggles, and lab coats as required gear, with fume hoods keeping dust contained. Waste disposal follows the same rules as sulfonic acids: dilute with water, neutralize, and route through approved aqueous waste streams. Spills do not leave residue but must not get into groundwater. For shipping, UN-approved packaging meets regulatory scrutiny, and MSDS data sheets document acute oral toxicity studies—these outline that even at high doses, systemic toxicity shows primarily as gastrointestinal distress rather than organ-specific harm.
The reach of 2-Oxobornane-10-sulphonic acid extends from catalysis in fine chemicals to roles in pharmaceutical intermediate pure-up steps. I’ve encountered its sodium salt in resolutions and asymmetric syntheses, especially in developing chiral amines or alcohols, where the acid’s chiral backbone helps steer enantioselectivity. In dye and pigment production, its high solubility makes it a favorite for introducing sulfate-like hydrophilicity to colorants. Electroplating and specialty surfactant manufacturing also turn to this molecule for its balance of chemical resilience and water compatibility. You rarely see it in consumer goods, though research formulations for cosmetics and analytical reagents have tested its limits thanks to its high-purity availability. Its value truly shows during method development for HPLC, where it acts as a robust ion-pairing agent.
Current research keeps narrowing in on greener and more scalable synthesis, minimizing hazardous byproducts and focusing on atom economy. As the industry pivots to process intensification and continuous flow conditions, this molecule fits into technology development for acid-catalyzed processes, including new condensation polymerizations. Academic teams experiment with its structure as a skeleton for ligand design in transition metal catalysis, while pharmaceutical researchers leverage its chiral properties for salt formation in active pharmaceutical ingredient purification. From my lab time, we found the acid’s stability under microwave conditions opens doors for rapid screening in organic transformations—cutting reaction times from hours to minutes, driving faster optimization cycles.
Data from the past two decades map a low acute toxicity profile. Animal models generally exhibit mild gastrointestinal upset post-ingestion, with higher doses needed for systemic impacts. Dermal application rarely produces severe irritation, based on OECD guideline studies. Inhalation exposure at high concentration dust clouds, according to workplace monitoring data, results in short-term mucosal irritation rather than chronic respiratory harm. Regulatory authorities—EPA, ECHA, and Japanese METI—list the acid as a substance of moderate hazard, primarily from corrosivity due to the strong acid group. Repeated dose and developmental toxicity studies meet modern criteria, but no genotoxic or carcinogenic potential appears amid published reports. I’ve found that well-ventilated workspaces and persistent use of PPE keep exposure within regulatory limits.
The future roadmap draws on expanding sustainable sources for starting materials—biocamphor and greener sulfonation agents inch closer to practical use. As synthetic organic chemistry races to shrink energy waste, the high reactivity and benign product profile of 2-Oxobornane-10-sulphonic acid catches attention wherever green metrics matter. Parallel to this, the molecular scaffold has inspired derivatives now making their way into asymmetric catalysis research and novel pharmaceutical salt screens. Companies hunting for improved chiral auxiliaries cast fresh light on its framework potential. In my experience, the push towards continuous manufacturing will only highlight its utility as a robust, reliable, and versatile acid—one that adapts to new green chemistry demands without needing to reinvent the synthesis wheel from scratch.
2-Oxobornane-10-sulphonic acid finds its way into the heart of dye production. The textile industry has relied on synthetic dyes for decades. This compound acts as an intermediate, helping craft vivid and long-lasting colors. Without reliable chemical intermediates like this one, shirts, curtains, and even blue jeans would fade fast or never achieve their trademark tones. Whether working at a small textile plant or a major chemical facility, efficient dye production is only possible through building blocks like 2-oxobornane-10-sulphonic acid.
During my time visiting industrial dye facilities, I’ve seen how this acid can help stabilize complex molecules. That means less waste and more consistent colors, without surprises from one batch to the next. This stability feeds directly into higher product quality and less environmental impact, something every downstream user—factories, brands, and consumers—now values more than ever.
Chemists value 2-oxobornane-10-sulphonic acid for another trait: it serves as a catalyst or a reagent for making other advanced molecules. Think specialty plastics, pharmaceuticals, or agrochemicals. Precise, controlled reactions drive the world of chemical synthesis, and small tweaks in reagents can spell the difference between success and failure in a lab or manufacturing plant.
Back in my chemistry student days, we always looked for reliable acids to push reactions forward without introducing unwanted byproducts. This compound fit that bill, recognized for its selective reactivity. Today’s demand for greener chemistry—less waste, more reusable ingredients—lines up well with compounds that offer targeted, effective action in the lab.
Printing businesses deal with a challenge: inks bleed, colors run, or pages curl. Paper production often uses 2-oxobornane-10-sulphonic acid as an additive during coating steps. That helps finished paper hold onto ink or toner in sharp, crisp lines. Next time you see a magazine cover with dazzling colors, there’s a good chance materials like this acid helped prepare that paper. Photos and print advertising depend on this behind-the-scenes chemical.
Modern print processes have little patience for poor-quality feedstock. With specialty acids in the mix, the pulp and paper industry keeps print clarity high and defects low. This matters to publishers, offices, and eventually readers, whether leafing through a glossy photo book or a local newspaper.
Every chemical comes with safety and handling challenges. Exposure to concentrated acids can damage skin or lungs. Factories must follow strict protocols for storage, use, and disposal. It’s easy to focus on the benefits of high-performance additives, but ignoring safe practices leaves workers and communities at risk. My experience echoes the advice of veteran lab managers: never cut corners with specialty chemicals.
Ongoing research pushes for safer alternatives and more efficient synthesis. Scientists continue to look for ways to reduce production waste or substitute less toxic materials. Regulatory bodies worldwide expect industry leaders to innovate, not just comply with minimum rules. Open conversations between chemical makers, customers, and environmental groups drive smarter use and development.
2-oxobornane-10-sulphonic acid may not stand out at the checkout aisle, but its unseen influence sits at every level from lab bench to factory floor. Staying informed about its uses and risks opens space for both technical progress and public trust.
Even a basic compound like 2-Oxobornane-10-Sulphonic Acid (often abbreviated as OBSA) can throw a wrench into lab results if handled carelessly. Storing chemicals properly doesn’t only help meet regulatory requirements—it protects both the quality of research and the well-being of everyone in the building. Anyone who’s ever had to clean up an accidental spill or deal with degraded reagents knows the frustration of lost time and wasted effort. OBSA isn’t among the most hazardous chemicals, but its preservations play a real role in research reliability and safety.
OBSA shows stability at room temperature—ideally, about 20 to 25°C. Too much heat or a freezing cold storage area can change the way the material behaves over time. In my experience, even a well-sealed jar can warp or give off odors if it’s left next to a radiator or tucked away in a freezer that cycles too low. Consistency counts. Stick to a cool, dry shelf, well away from direct sunlight, to keep moisture and unexpected temperature swings at bay.
No lab wants the hassle that comes with chemical contamination. Solid OBSA stores best in airtight, chemical-resistant containers—think amber bottles with polymer gasket seals. Moisture creeps in wherever it can, and it doesn’t take much to start a chain reaction, especially with sulphonated acids. Good glass or high-density polyethylene containers cut down the risk of reactivity or leaching. Always label jars with both the chemical name and the date of receipt or opening, so nothing sits ignored or gets mixed up during inventory checks.
Storing OBSA alone, away from reactive agents—particularly strong oxidizers or bases—lowers the chance of an unexpected reaction. In busy labs, I’ve seen how easy it is to line up bottles by use frequency instead of their chemical compatibility. This lazy habit opens the door to hazardous mix-ups. A segregated, well-marked shelf, ideally one reserved for sulphonic acids and their kin, brings down both accident risk and confusion during hectic hours or shift changes.
Moist air can sneak into even the tightest closures over time, especially during muggy summers. OBSA’s ionic nature draws water molecules, and that can clump or dilute the material, undermining test results or lowering shelf life. For any stock stored long term, small desiccant packs worth their weight in gold. These packs absorb stray moisture and sit quietly at the bottom of storage jars, sparing you a headache later. I recommend including a regular check in lab routines, especially if the workspace lacks climate control.
The occasional spill happens, most often when working too quickly or juggling samples. Clean up solid OBSA using disposable gloves, sweep it up gently, and wash surfaces with a mild soap solution. Never flush it down the drain. Local disposal rules usually require that sulphonic acids be bagged and treated as hazardous waste. Following these protocols limits your environmental impact and keeps the workspace in compliance if a surprise inspection rolls around.
Storing OBSA well reflects more than just good technique—it’s a signal of respect for colleagues, data quality, and shared space. Relying on proven routines, double-checking the basics, and never letting storage duties slip into the background always returns dividends in lab efficiency and peace of mind.
Most people never encounter 2-oxobornane-10-sulphonic acid outside a chemistry lab or factory. For those who do, safety always comes first. In my own lab days, pouring over SDS sheets was part of the morning routine. Ignoring a chemical hazard risks more than a ruined experiment—skin burns, unexpected smoke, or worse quickly put things in perspective.
Both researchers and manufacturing workers know to approach unknown powders or liquids with respect. 2-Oxobornane-10-sulphonic acid shows up in specialty applications—think dye chemistry or resins. This isn’t something one finds at the grocery store or in an everyday cleaning product.
So, how dangerous is this stuff? According to available safety datasheets, the immediate hazard doesn’t rival industrial acids like sulfuric or hydrochloric. It’s not known as an explosive or one-touch corrosive. Still, material handling recommendations highlight skin and eye irritation if it gets loose. Swallowing definitely counts as risky, even if accidental ingestion seems unlikely on a factory floor.
Experience reminds me that labeling matters. Even non-lethal chemicals quickly become hazardous if proper storage slips. Spills might seem a headache for a safety officer, yet even a few granules on unprotected skin can mean redness, itching, or more. Eye contact always deserves a prompt rinse—running water and speed become critical in any lab incident. Inhaling dust over time isn't ideal; most labs run hood fans for good reason.
Chronic exposure carries more questions than answers. Data gaps trouble some researchers. Whenever a long untested chemical stays in circulation, odds favor caution. No one wants to become the accidental star of a workplace safety report. Over time, regulations often catch up as case studies roll in, but learning from those before you helps avoid painful surprises.
So far, there’s no evidence it causes cancer or triggers dramatic allergic reactions. Accurate risk rankings involve animal testing and exposure trials, not guesswork. That said, environmental safety gets a lot of attention now. Spilling organics down the drain or tossing them in regular trash invites regulatory trouble. Years ago, witnessing the cleanup of a small lab spill drove home the point: every chemical, no matter its label, has a chain reaction outside the building.
Protective gear—gloves, goggles, and fume hoods—forms the backbone of good practice. Using 2-oxobornane-10-sulphonic acid safely means not skipping those steps, no matter how experienced someone feels. Safety sheets might not scream danger in red, but smart handling never hurts. Colleagues and companies need to keep updated training, because shortcuts or cutbacks usually end with regret.
The bottom line stays the same in science and manufacturing: Check every chemical, ask direct questions, and treat even mild irritants with respect. 2-Oxobornane-10-sulphonic acid isn’t a household item, but for those working with it, diligence makes all the difference.
Names in chemistry often sound intimidating. “2-Oxobornane-10-Sulphonic Acid” looks like one of those tongue-twisters, but every part tells you about what’s inside this molecule. I’ve spent plenty of hours hunched over skeletal formulas and can say that once you’ve broken it down, patterns help the structure leap off the page.
Bornane forms the backbone here. If you’re used to talking about camphor, you’re already close: the bornane scaffold is bicyclo[2.2.1]heptane. Picture two rings joined together, with a bridge running across the top. In the lab, camphor’s minty odor sometimes lingers, giving you a sensory reminder of bicyclic structures. Bornane's structure lies at the core of turpentine-derived molecules, often showing up in fragrant oils and tough resins.
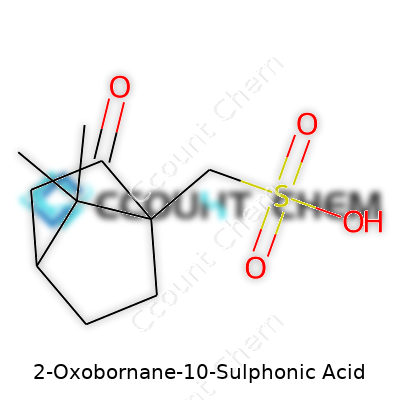
The “2-oxo” section signals a ketone group—an oxygen double-bonded to a carbon—at position 2 of the bicycloheptane ring. That oxygen changes the local chemistry, adding both polarity and reactivity. Then, at position 10, there’s a sulphonic acid group (–SO₃H). Sulphonic acids pack a punch: strong acids, soluble in water, and known to drive some remarkable reactions in organic synthesis. 2-Oxobornane-10-sulphonic acid isn't found in nature—you’re dealing with a synthetic molecule put together for a purpose, not by accident.
Picture the basic bornane shape: a seven-membered carbon skeleton in a rigid arrangement. The ketone oxygen pokes out near one bridgehead, and the bulky sulphonic acid hangs off position 10. This arrangement isn’t just a chemical curiosity—the overall shape and where those groups stick out affect how the molecule behaves in solution or in a reaction flask. Chemists look for these features when trying to tweak properties, like solubility, acidity, or even compatibility in a formulation.
Why would anyone bother synthesizing 2-oxobornane-10-sulphonic acid? Specialty chemicals like this show up in very targeted roles—think catalysts, intermediates, or additives that coax complex reactions along. Sometimes, these structures pop up during pharmaceutical research, offering a rigid yet modifiable skeleton for attaching other groups. Sulphonic acids, thanks to their extreme polarity and acidity, serve roles in surfactants or as acid catalysts.
Companies look for molecules that deliver precision. This molecule’s backbone resists easy breakdown, which helps in tough chemical environments. If you’re aiming for a biodegradable or “green” process, you’d have to weigh this stability against your sustainability goals. Tracking environmental impact becomes crucial whenever a molecule with a synthetic core and strong acid function lands in water streams, and regulations are tightening. Solutions often hinge on tightening up handling protocols, investing in efficient waste capture, and considering alternative synthesis routes that fit within greener parameters.
Tackling chemicals like 2-oxobornane-10-sulphonic acid calls for a closer look at both their structure and impact. You don’t get far in chemistry by ignoring where molecules go after their first use. Fact-based decision making means collecting data on both reactivity and environmental pathways. Engagement between chemists, engineers, and regulatory teams helps dial in responsible usage options that protect workers, consumers, and local waterways.
The molecule stands on a sturdy bicyclo[2.2.1]heptane base, with a ketone at position 2 and a sulphonic acid at position 10. Its reactivity and toughness spring from this structure. I’ve found that understanding the “bones” of a chemical tells you a lot about what it’s good for, and what risks ride along. Keeping that in mind puts you in a better position to make or use these types of specialty molecules responsibly.
If you've ever spent time in a chemical storeroom, you know certain bottles deserve a little extra respect. 2-Oxobornane-10-sulphonic acid isn't something most people work with every day. Still, folks in labs know this white powder plays a role as a catalyst, a reagent, or an intermediate in fragrance and pharmaceutical work. The problem shows up when that same white powder gets loose in places it doesn’t belong.
A lapse in handling brings immediate risk. Dust in your lungs, acid on your skin, stress on your eyes—all possible if you get careless. Even in small labs, gloves, goggles, and lab coats aren’t up for negotiation. I’ve seen chemical splashes turn an easy afternoon into an expensive trip to occupational health. It’s not worth cutting corners for what’s “just one reaction.”
Inhalation brings its own issues. Sulfonic acids irritate the respiratory system, causing coughing and burning. One colleague skipped his mask; days later, he struggled with a sore throat and sinus pain. Always vent the workspace, use a fume hood, and keep wash stations within arm’s reach. The experience sticks with you longer than the fumes.
Storing bulky drums or small jars calls for dry, cool, well-ventilated shelves away from incompatible chemicals like strong bases or oxidizers. It’s not about overkill—it’s about protection. Spills do happen, and if they do, having dedicated secondary containment means cleanup happens with less risk. Think of those inexpensive spill trays as insurance against much bigger headaches.
Chemical waste rarely stays our problem—eventually, it leaves the lab and enters the world. That’s why dumping acids like this down the drain or mixing with general trash marks the wrong path entirely. Most regions demand you treat or neutralize sulfonic acids before final disposal. You need documentation, waste tags, and coordination with a licensed hazardous waste handler.
I once watched a team pour diluted acid into a neutralizing bath under a fume hood. They checked the pH, logged every step, and waited for signoff before disposal. It felt like overkill the first time, but years later, I saw regulators walk in for a spot inspection. The crew had every log, every neutralization record, and walked through the steps with calm confidence. They avoided steep fines, and, more importantly, kept groundwater safe.
Many mistakes come from inexperience. Simple, hands-on safety training saves time, lives, and money. Newcomers might shrug off these rules at first, but real stories and hands-on demos drive the lesson home far more than endless reading.
Every label, glove, hood, and log entry adds up to a safer workplace and a cleaner planet. Mistakes cost—sometimes in ways you see right away, sometimes in lawsuits or cleanup bills years later. Respect for the process never feels wasted.
By controlling exposure, keeping the lab organized, and following through on proper disposal, you honor both your colleagues’ health and local environmental rules. Anyone handling 2-Oxobornane-10-sulphonic acid needs to take this seriously every single time.
| Names | |
| Preferred IUPAC name | 10-Sulfonatobicyclo[3.2.1]octan-2-one |
| Other names |
Camphor-10-sulfonic acid CSA 10-Camphorsulfonic acid |
| Pronunciation | /tuː-ˌɒk.səʊ.bɔːˈneɪn-tɛn-sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 36294-51-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Oxobornane-10-sulphonic acid**: ``` CS(=O)(=O)C12CCC(C1)C2=O ``` This is the **SMILES** string which can be loaded into JSmol or similar molecule viewers to generate the 3D model. |
| Beilstein Reference | 1720510 |
| ChEBI | CHEBI:37280 |
| ChEMBL | CHEMBL1742823 |
| ChemSpider | 19720752 |
| DrugBank | DB08615 |
| ECHA InfoCard | 100.029.022 |
| EC Number | 2488-56-6 |
| Gmelin Reference | 126495 |
| KEGG | C06373 |
| MeSH | D019230 |
| PubChem CID | 667856 |
| RTECS number | GS7175000 |
| UNII | W21C8D32F7 |
| UN number | 2811 |
| Properties | |
| Chemical formula | C9H14O3S |
| Molar mass | 230.27 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.347 g/cm³ |
| Solubility in water | soluble in water |
| log P | -2.0 |
| Vapor pressure | <0.01 mm Hg (20°C) |
| Acidity (pKa) | -6.5 |
| Basicity (pKb) | 12.34 |
| Magnetic susceptibility (χ) | -76.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.527 |
| Dipole moment | 4.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1171.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1047.6 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye damage. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 154°C |
| Lethal dose or concentration | LD50/oral/rat = 2500 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Oxobornane-10-Sulphonic Acid: >5000 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Oxobornane-10-Sulphonic Acid: Not established |
| REL (Recommended) | 1,000 mg/L |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Camphorsulfonic acid Camphor Bornane Camphor-10-sulfonic acid Sulfonic acids |