In chemical history, 2-hydroxyethanesulphonic acid gained traction as early industrial chemistry stretched beyond simple acids and bases to specialty compounds suited for niche roles. The compound stepped into the spotlight during the surge in dye and pharmaceutical synthesis in the 20th century. Researchers, pushing for new sulfonic acids with improved water solubility and distinctive reactivity, explored the straightforward two-carbon backbone with a sulfonic acid and a hydroxy group. Personal experience in university labs introduced me to its manageable acidity and surprising chemical resilience, which underlining why specialty acids moved beyond mere laboratory curiosities. Its adoption often meant chemists no longer struggled with poor solubility or unpredictable side reactions—an issue that plagued earlier sulfonic acids.
2-Hydroxyethanesulphonic acid typically shows up as a clear, slightly viscous liquid or a colorless solid. Chemical suppliers offer it in various concentrations, with tight attention to purity. The market usually recognizes it under names such as Isethionic acid or hydroxyethanesulfonic acid. It belongs to an overlooked family of compounds that bridges the space between small-molecule reagents and performance additives. In day-to-day laboratory settings, people lean on this compound when they want an acid with gentler handling requirements, compared to some alternatives. Working with it feels less risky than some of its stronger counterparts, allowing greater flexibility with storage and transportation.
The acid carries the molecular formula C2H6O4S, packing both a hydroxyl and a sulfonic group onto a two-carbon chain. This brings a melting point around 100°C and a typical appearance as a solid at room temperature, shifting to syrupy liquid with modest heating. Its strong acidity mixes with pronounced hydrophilicity, which explains frequent use in aqueous solutions and its ready dissolution in water. Chemically, it stands robust against moderate heat and most oxidizers, resisting decomposition unless subjected to harsh conditions. With personal use in buffer preparations, it rarely disappoints because it dissolves instantly in standard lab water and shows minimal interference with many analytical methods.
2-Hydroxyethanesulphonic acid shipped for the lab or industry arrives with careful labeling that spells out concentration, purity (commonly over 99% for high-grade material), and batch certification. Packaging guidelines trace back to international demand for clarity. Labels show hazard pictograms as dictated by GHS, clear indications of storage conditions, and any applicable regulatory compliance numbers. As someone who has scrutinized bottles on the shelves, clear lot traceability on every drum or ampoule builds trust. Handling sheets typically rest next to product containers, referencing minimum safe handling practices.
Chemists usually turn to the classic method of reacting ethylene oxide with sodium bisulfite in aqueous medium, yielding sodium isethionate, which then undergoes acidification with a strong acid like hydrochloric to free the 2-hydroxyethanesulphonic acid. Scale-up over the years demanded careful temperature control and scrupulous purification, given the tendency for side reactions or the formation of colored byproducts if conditions wander. Many midsize processors have refined this with automated reactors, creating lots that meet the needs of pharmaceutical and food sectors, constantly checking for trace contaminants. My own small-scale syntheses started with the textbook reaction, and hitting reliable yields usually came down to precise temperature and constant stirring.
2-Hydroxyethanesulphonic acid stands out for its participation in mild esterification reactions, and many chemists rely on its hydroxy group to tether small molecules or in modifying surface properties. The acid group holds up to moderate heating and mild reducing agents, making it a go-to for synthesis requiring a controlled degree of acidity. Sulfonate esters derived from this molecule have surfaced in surfactant chemistry, where they contribute to wetting properties and stability. Reactions with alcohols, epoxides, or amines offer paths to secondary derivatives, each tweaking its utility for new applications. In my time collaborating with surfactant chemists, 2-hydroxyethanesulphonic acid cropped up repeatedly in discussion—its mix of manageable reactivity and safe profile cannot be overstated.
The compound often appears under various monikers—besides 2-hydroxyethanesulphonic acid, its labels may read isethionic acid, hydroxyethylsulfonic acid, or even under trade-specific names borrowed from food or pharma suppliers. Each variation signals to users its potential grade or purity, catering to those searching for the cleanest form for sensitive synthesis or broader grades for industrial blends. In reviewing catalogs, minor regional shifts in nomenclature sometimes confuse, but cross-referencing with CAS numbers clears uncertainty.
Standard protocols recognize 2-hydroxyethanesulphonic acid as a low to moderate hazard. Eye protection, gloves, and lab coats protect against accidental splashes, with well-ventilated storage in plastic or glass containers to ward off slow container corrosion. The acid itself does not vaporize at room temperature, so inhalation risks drop compared to volatile acids. Suppliers routinely ship it with safety data sheets outlining first aid and spill cleanup, ensuring even modest setups can respond quickly to incidents. In my regular lab routines, careful handling foregoes issues—splash incidents are rare, and mild irritancy means even accidental contact is handled promptly with water rinsing.
This acid weaves into a surprising variety of industries. In the pharmaceutical sector, it stabilizes formulations or supports reactions demanding unintrusive counterions. In cosmetics, it regulates pH and enhances solubility, giving lotions, shampoos, and cleansers smoother consistency. Food scientists, strangely enough, use it to adjust acidity or enhance shelf life, counting on its non-toxicity, which regulatory agencies support. Electroplaters eyed it for electrolyte baths, finding it beneficial for creating uniform metal layers. Across cleaners and surfactants, its salts offer foaming and cleansing without harsh after-effects. My visits to process plants show the acid handled in both batch and continuous flow lines, testifying to its popularity with technical personnel.
Current research circles often look for greener and more cost-effective production. There's growing interest in integrating bio-based raw materials or co-synthesis with other common sulfonic acids to cut waste. Project groups in universities partner with process engineers, exploring continuous flow reactors and alternative solvents to raise efficiency. Other labs explore functionalizing the molecule for new surfactant architectures—especially biodegradable versions—where government and consumer demand align. Synthesizing hybrids with other functional groups opens new doors for drug development or specialty coatings. In my own network, discussions with early-career researchers repeatedly pin 2-hydroxyethanesulphonic acid as a starting block in projects chasing both environmental gains and better product performance.
Toxicologists generally consider 2-hydroxyethanesulphonic acid a compound with low acute toxicity. Rodent studies back its safety profile, and chronic exposure reviews suggest minimal risk when used within established guidelines. Food and cosmetic regulators track intake levels, enforcing strict limits but often allowing use where other acids cannot go. Skin contact may lead to mild irritation, but no evidence points to systemic toxicity from normal applications. For years, my own exposure in university and industrial settings matched this consensus—occasional accidents caused brief discomfort but never long-lasting harm. Ongoing research surveys its breakdown products and environmental fate, ensuring future use remains within safe boundaries.
Looking ahead, 2-hydroxyethanesulphonic acid stands poised for further adoption across personal care, pharmaceuticals, and green chemistry. As supply chains circle back to sustainability, the simplicity and low toxicity of this molecule play to its advantage. Researchers eye it for next-generation surfactants made from renewable resources and as a base molecule for medical devices or battery electrolytes. There's excitement about new preparation methods cutting both cost and environmental impact, perhaps involving enzymatic routes or advanced catalysis. Industry watchers expect quality standards to tighten, with more traceability and eco-certification as buyers demand transparency. Conversations I’ve had with chemical producers always circle back to finding safer, more efficient acids for both legacy and emerging technologies, and this compound almost always has a seat at that table.
Few people outside science circles recognize the name 2-Hydroxyethanesulphonic acid, but you’ll find it behind the scenes in several industries. It goes by another name—isonipecotic acid’s cousin, ethanesulfonic acid—and shows up in places most folks never think to look.
In chemistry labs, it’s a buffer staple. During my time working on enzyme reactions for a university project, I learned to appreciate its reliability. Researchers reach for it because it keeps pH stable—a vital job during all sorts of metabolic studies. Without steady pH, reaction results zigzag, wasting time and resources.
You’ll spot it in HPLC (high-performance liquid chromatography) too. Scientists use it to help separate and measure different compounds in a sample, especially ones that won’t cooperate with other acids or buffers. It’s not glamorous, but crucial work often isn’t.
Pharmaceutical manufacturers lean on this acid in the development and purification of drugs. My pharmacist friend tells me that it’s useful for crafting water-soluble drug salts—think of meds that dissolve quickly and deliver active ingredients promptly. Without helpers like 2-Hydroxyethanesulphonic acid, some treatments would take too long to work or might not work at all.
Beyond solubility, drug researchers need their products to remain stable. Sulfonic acids help medicines stick around on the shelf, so patients and clinics don’t have to toss out medicines before the label says so. While consumers rarely see the chemistry at play, the results trickle down to lower waste and better access.
Walk into a paper plant, and you’ll find all kinds of acids. Many years ago, on a tour of a pulp and paper mill, I spotted barrels marked with names like methanesulphonic and ethanesulphonic acids. Here, 2-Hydroxyethanesulphonic acid helps clean and bleach wood pulp. Stock must be bright and pure for quality paper, and the chemical’s ability to break down contaminants speeds up production.
It’s also a go-to additive during textile printing, dyeing, and leather tanning. Ask anyone who’s tried to color fabric how important it is to get a sharp, vivid finish—a weak acid slows the process and leaves the colors looking tired.
Chemicals deserve respect, not fear. Most workers who handle 2-Hydroxyethanesulphonic acid know to wear gloves, goggles, and use proper ventilation. Industry guidelines stress containment, storage, and handling by trained staff. Decades of research and accidents have taught companies to pay attention.
Environmental regulations keep its disposal in check. Manufacturers now focus more on byproduct treatment and waste scrubbing. In my community, local ordinances require chemical users to submit annual environmental impact reports, spurring companies to rethink how they manage acids so rivers, soil, and air stay safe.
Some startups explore alternatives that blend safety and efficiency. Others improve recovery methods, squeezing more use out of every kilogram. That constant search for cleaner, cheaper, and safer chemicals shapes the future of industry—2-Hydroxyethanesulphonic acid included. Solutions come from combining experience with a willingness to try better ideas. In the end, understanding one chemical’s journey from lab bench to medicine shelf or factory floor can open eyes to just how much careful chemistry supports modern life.
2-Hydroxyethanesulphonic acid goes by several names, but most chemists recognize it as Isethionic acid. Its formula reads C2H6O4S. The molecule starts with a two-carbon backbone—think ethane—hooked to both a hydroxyl group and a sulfonic acid group, one on each end. Its full structure looks like HO–CH2–CH2–SO3H. The sulfonic acid group brings strong acidity, much like you see in sulfuric acid, making this molecule soluble in water and reactive where you want strong acid action in a mild context.
Isethionic acid doesn’t stand out in everyday life like ascorbic acid or citric acid, but its fingerprints show up in personal care. Surfactant manufacturers use it as a building block for mild, skin-friendly soap alternatives. It forms sodium isethionate, which ends up in body washes touted as gentle, unlike harsh sulfates that can strip protective oils from skin. Chemists favor isethionic acid because that sulfonic acid group combines with the hydroxyl side for stability and reactivity in one package.
Having worked with chemical formulations, it’s clear isethionic derivatives bring practical benefits. They hold up under a range of pH values and don’t irritate like older cleaning agents did. Think about washing your hands dozens of times. The skin comfort difference traces back to sulfate-free surfactants, many built off isethionic acid.
One of the concerns in modern chemistry is what happens to these chemicals down the drain. Isethionic acid salts biodegrade more easily than traditional surfactants. They break down under wastewater treatment, leaving fewer worries about residues in rivers. Regulatory agencies in Europe and North America look favorably on formulations with this chemistry for precisely these reasons. Fewer complaints about skin irritation also show up when product testers rate isethionate-based cleansers.
On safety, isethionic acid itself is considered lower in toxicity compared to legacy cleaning agents like sodium lauryl sulfate. Studies support this, showing minimal impact when diluted or neutralized. For formulators aiming for high safety profiles, it allows creative freedom without running afoul of restrictions or causing allergic reactions in consumers. Many labs keep a bottle on the shelf because it works without causing trouble.
The science around isethionic acid keeps moving. Production can lean more sustainable, cutting down on petroleum by improving synthesis from bio-based feedstocks. Waste reduction also benefits from better yield processes. Safe doesn’t always mean perfect, so periodic toxicity screening makes sense. Focused research helps spot rare sensitivities before products launch in bulk.
As demand for “greener” and safer ingredients grows, companies keeping up with both supply chain transparency and laboratory testing get ahead. Chemists should share insights with product developers and regulators, so everyone trusts what lands on the shelves and eventually in household water streams. It’s one molecule in a broad toolkit, but 2-hydroxyethanesulphonic acid opens the door for smart, practical chemistry many of us rely on without knowing it.
2-Hydroxyethanesulphonic acid—also called Isethionic acid—turns up in a surprising range of jobs. Some labs use it in electroplating solutions. Others see it in textile dyeing or as a building block for surfactants and detergents. Plenty of folks shrug off most chemicals as if a glove and a good sense of direction will do, but some compounds ask more of us. This is one of them.
Touching 2-hydroxyethanesulphonic acid with bare hands can burn your skin. Splash it near your eyes—damage is likely. Breathing vapors, particularly when heating or mixing, can irritate your airways. Spills feel minor at first: an accidental drop splatters on the floor, and the urge is to wipe it up quickly and move on. Later, painful redness can spread, reminding you that strong acids always leave their mark.
Anyone who’s cleaned a drain with off-the-shelf acid feels the sting of bad technique. My own run-in came from thinking a tiny amount on my finger would just dry up on its own. It did not. Friction and sweat only opened the skin wider, making the lesson stick. The label’s hazard pictograms—corrosive, irritant—mean something if you let them.
Manufacturers offer Safety Data Sheets on every shipping box, which you should read and not just sign off. According to research, exposure to 2-hydroxyethanesulphonic acid causes eye and skin burns. If inhaled as a mist, it inflames the nose and throat. Chronic skin contact can lead to persistent skin problems. Accidental mixing with other chemicals sometimes sets off nasty, unplanned reactions.
The American Conference of Governmental Industrial Hygienists reports that acids of this type should always be considered hazardous to health on contact. The risks go beyond isolated burns; over time, inadequate safety culture in any workplace stacks up, lowering morale and raising the odds of serious injury.
In my past lab roles, the rule always held: prepare for the worst and don’t rush. A splash-proof lab coat, gloves rated for chemical resistance, and goggles are not optional—they replace regret. Fume hoods or working in open, airy spaces help sidestep accidental inhalation. Even experienced technicians double check lids and wipe down bottles before moving them between benches.
Never eat or drink in the work area. Store acids in strong containers, not next to bases or organic solvents, and monitor storage conditions. Always mark bottles with clear hazard labels; in one job, faded markers confused a coworker into grabbing acid instead of a buffer. Emergency eyewash stations should be checked every week. If a splash happens, no one wastes time—fifteen minutes minimum at the wash, then straight to occupational health.
Disposal asks for a methodical touch. Neutralize the acid using a proper base (not just whatever is closest), then follow local hazardous waste rules. Never dump anything corrosive down the drain—pipes and the water table pay for that mistake.
Safety with 2-hydroxyethanesulphonic acid comes down to respect. Gloves and goggles cost pennies compared to the pain of a trip to the ER. Read the labels, check your gear, and make responding to mistakes second nature. The stories behind bad chemical burns aren’t usually heroic or unique; most start with someone thinking, “It’ll be fine this time.” It rarely is. Keeping sharp and taking chemicals seriously keeps you, your lab, and everyone around you out of trouble.
2-Hydroxyethanesulphonic acid has a reputation for reliability in many chemical and industrial labs. From cleaning agents to electroplating, people trust it for the way it functions. Chemical safety rarely shows up in the spotlight, but skip the basics, and an accident soon knocks at the door. This acid behaves predictably—until it doesn’t, once heat or contamination play their parts. Its structure, built around a sulfonic acid group and a hydroxyl group, holds up well under normal conditions. Yet, in my experience, good chemistry often falls apart because someone overlooked storage basics. Simple choices, such as where to put the drum or bottle, set the stage for safety or risk.
Too many labs try to save space by stacking acids in random corners. Over time, even a compound as stable as 2-hydroxyethanesulphonic acid shows changes if left in the sunlight or next to a heat source. At higher temperatures, chemical degradation speeds up. The acid’s active properties can weaken, and decomposition byproducts might form, sometimes without obvious warning signs. I’ve seen labs ignore cracked or foggy containers, only to later discover sludgy residues.
Direct light makes things worse. Ultraviolet exposure can encourage unwanted side reactions. Any technician who’s spent late nights cleaning up sticky messes from burst bottles knows how much hassle this brings. Use brown glass or opaque plastic containers, and keep them in a dark storage cabinet.
Anyone who’s handled acids knows water acts as both friend and enemy. With 2-hydroxyethanesulphonic acid, even trace moisture from humid room air helps dilute the acid, and sometimes surprises appear as white crystals or unexplained thinning. Impurities find their way in too, whether from a dirty scoop or careless pouring. Cross-contamination doesn’t just waste chemicals. It eats into research budgets and spoils entire batches.
Tight lids and proper seals matter. Polyethylene and glass containers work well, as they resist corrosion and don’t react with the acid. You’ll see fewer leaks, fewer markings on shelves, and fewer angry calls from inventory managers if you pay attention to these details. Label every bottle clearly—unmarked acids create chaos and risk.
The most reliable results come from simple routines. Regularly check the date of purchase and the appearance of each container. Do not let old stock hang around for years. If liquid looks cloudy or the container turns sticky, treat it as a warning sign.
Spill kits, eye wash stations, and gloves should never gather dust. Frequent training goes a long way: lab staff and warehouse workers need reminders on handling and the risks of improper storage. It helps to post checklists near shelving or inside chemical storerooms. Periodic audits have saved my teams hours of crisis management by catching careless mistakes early.
Industry groups and chemical safety bodies repeatedly point towards cool, dry, dark storage as the best bet for organic sulfonic acids. OSHA, NIOSH, and European agencies echo each other’s advice. Following these guidelines does more than keep you out of regulatory trouble. It means that even if the power fails or an unexpected delivery arrives, your stock of 2-hydroxyethanesulphonic acid stands a better chance of getting through in usable condition. Careful storage isn’t just theory—it keeps people safe and equipment intact.
If you’re in the market for 2-Hydroxyethanesulphonic Acid—often called Isethionic Acid—your search might start online, but shopping smart means digging deeper. Plenty of big-name chemical suppliers like Sigma-Aldrich, Thermo Fisher, and Merck list this compound. These trusted companies keep this acid in regular supply thanks to strong industrial demand, especially for detergents, cosmetics, and pharmaceuticals.
Local distributors fill a similar role and keep the process simple, especially for business buyers. For anyone looking to buy on a smaller scale, a local chemical distributor is worth a phone call. If your needs grow or you want a more direct line, working with manufacturers in regions like China or India can drive better prices. Just keep in mind: due diligence counts here. Ask for a certificate of analysis, check the supplier’s reputation, and don’t brush past regulatory paperwork. I learned this the hard way after one seemingly “cheap” shipment got caught by customs for missing RoHS paperwork—it delayed our R&D work by weeks.
Packaging matters, and not just because of shipping costs. The acid typically comes as a white crystalline powder or a liquid, so suppliers offer options from tightly sealed bottles to robust bags and industrial drums. For small laboratory use, high-density polyethylene (HDPE) bottles protect the contents and minimize the risk of leakage—a necessity for labs I’ve worked in, where only a few hundred grams get used in a month.
Bulk buyers live in a different world. Here, you start seeing fiber drums lined with polyethylene, or stainless steel drums for large orders. Fifty-kilogram drums are the norm for product blending or large-batch production. Some suppliers use double-layered plastic bags inside cardboard barrels, which gives extra protection during transport. Liquid forms call for thick-walled canisters or barrels to prevent leaks. For shipments headed overseas, many opt for intermediate bulk containers (IBCs), which make transferring the acid safer and more cost-effective during transit. No one wants to clean up a spill at the port—trust me.
Knowing exactly where and how much to order does more than save money. Product purity, packaging quality, and supplier reliability have a direct impact on downstream processes. Contaminated or poorly packaged acid can mess up product batches, cause corrosion in storage, or result in fines during import. Sourcing from established suppliers also means batch traceability, so if something goes wrong, you can track the issue at its root.
It’s smart to weigh supply chain risks and local availability. Keeping a local backup supplier is a lesson I picked up during the pandemic, where global delays turned common chemicals into rare commodities overnight. If you run a business relying on steady supplies, set up recurring orders and keep some inventory on hand. A trusted relationship with a supplier can help you navigate emergencies and special requests.
Regulations keep tightening to protect people and the planet. Stick to suppliers who clearly state compliance with REACH, RoHS, or ISO standards. If in doubt, request supporting documentation. Some companies even provide batch-level traceability, so you can verify each shipment’s journey and its quality. A solid reputation, clear labeling, and support from skilled personnel go a long way in chemical purchasing.
2-Hydroxyethanesulphonic Acid may seem like just another industrial chemical. In reality, smart buying decisions make all the difference between smooth operations and costly setbacks. Build connections, stay informed, and choose quality over a quick bargain to keep your projects running strong.
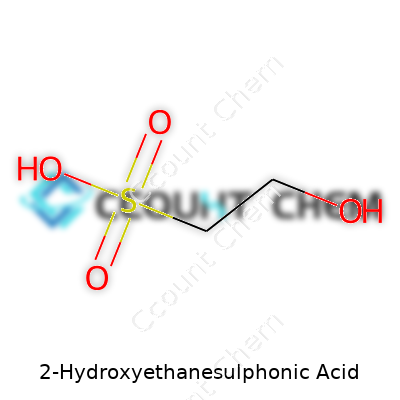
| Names | |
| Preferred IUPAC name | 2-hydroxyethane-1-sulfonic acid |
| Other names |
Isethionic acid 2-Hydroxyethanesulfonic acid Isethionique acid Isethionate 2-Hydroxyethane sulfonic acid |
| Pronunciation | /tuː haɪˌdrɒksiˌɛθeɪnˈsʌlfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 107-36-8 |
| Beilstein Reference | 1209371 |
| ChEBI | CHEBI:52743 |
| ChEMBL | CHEMBL1232 |
| ChemSpider | 38405 |
| DrugBank | DB04095 |
| ECHA InfoCard | '03b1d86f-9b70-40b6-bb3c-04b891cdcc99' |
| EC Number | EC 200-051-6 |
| Gmelin Reference | 4456 |
| KEGG | C02371 |
| MeSH | D017250 |
| PubChem CID | 6137 |
| RTECS number | KA3850000 |
| UNII | E3SO1D70TL |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID7020640 |
| Properties | |
| Chemical formula | C2H6O4S |
| Molar mass | 126.13 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.28 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.0 |
| Vapor pressure | 0.02 hPa (20 °C) |
| Acidity (pKa) | 1.53 |
| Basicity (pKb) | 1.01 |
| Magnetic susceptibility (χ) | -9.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.438 |
| Viscosity | 10 cP (25 °C) |
| Dipole moment | 3.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 239.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -888.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -964.8 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | A09AB16 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H290, H318 |
| Precautionary statements | P280, P305+P351+P338, P310, P303+P361+P353, P363, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 90 °C |
| Autoignition temperature | 460 °C |
| Lethal dose or concentration | LD50 Oral - Rat - 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2200 mg/kg (Rat, oral) |
| NIOSH | KM8050000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) of 2-Hydroxyethanesulphonic Acid: "5 mg/m³ |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Ethanolamine Ethanesulfonic acid Isethionate Taurine |