Chemistry keeps rolling forward, dragging old textbooks and fresh patents behind it. Somewhere along the way, 2-dimethylamino-1-hydroxy-ethanesulfonic acid natrium salt cropped up in catalogs. It didn’t show up by accident. Researchers have spent decades working with amino alcohols and sulfonic acids because those functional groups bring real muscle to buffering systems, biochemistry, and analytical setups. The introduction of this compound came as labs searched for tight pH control around neutrality, especially when studying enzymes that don’t take kindly to extremes. The march from early, crude buffers to smarter, less interfering compounds owes a lot to the patient, gritty work of trial-and-error from countless biologists, chemists, and instrument engineers.
This sodium salt behaves like a strong-willed buffer, holding onto a narrow pH window in experiments where even a tiny swing throws everything off. Its structure sports a dimethylamino group beside a hydroxyethyl backbone and a sulfonate anchor—enough to make any chemistry major sit up. Its main calling lies in labs: biopharma, clinical research, and basic science all rely on consistent solutions that don’t mess with the task at hand. Where proteins, nucleic acids, or sensitive cell cultures need a safe hand, this compound delivers.
2-dimethylamino-1-hydroxy-ethanesulfonic acid natrium salt usually appears as a crystalline powder—white or nearly so. It dissolves well in water, less willingly in organic solvents. The sodium cation helps steer its solubility, pulling the compound right where it’s needed in aqueous setups. Its buffering range hovers near neutrality. From practical experience, it seems stubborn under normal storage but appreciates a dry, closed bottle lest moisture and air spoil its character. Labs pay attention to its stability across time and temperature; breakdown products can confound data, so that consistent lot quality matters more than many folks realize.
I look for purity above 99%, since impurities seem to creep into every experiment. Most packaging provides the hydrate state, molecular weight, precise pH range, and any batch-specific data like trace metal content. Barcode tracking, QR codes, and compliance marks (such as ISO or GMP) increasingly find their way onto bottles. Good suppliers publish their analytical techniques—NMR, IR, and mass spectrometry—not just because it sells product but to give trust to the lab tech relying on each scoop. A slip here opens the door to faulty experiments or even recalled research, and no one wants those headaches.
Manufacturers usually synthesize the molecule by reacting the parent hydroxyethanesulfonic acid with dimethylamine under controlled conditions, then neutralize the product with sodium hydroxide. Practical yields and purity depend on temperature monitoring, careful pH control, and skilled handling of intermediates. Experienced chemists know water content at every stage can steer, or spoil, the reaction; drying steps, filtration, and recrystallization play a big role. Commercial labs prefer routes with fewer steps and easy purification, to cut costs and waste. Environmental rules have tightened, so green chemistry methods—solvent recycling, minimizing byproducts—carry more weight every year.
The molecule’s main chemical flexibility sits with its amino and hydroxy groups. Under the right pH, chemists can tweak its substitution pattern, attach labels for tracking, or cross-react it with other bioactive molecules. These modifications open up custom buffer systems or specialized test environments. In some research, the compound serves as a launching pad for even more complex molecules, especially for cell imaging or drug delivery studies. Anyone tampering with the core structure must respect how changes can shift solubility, toxicity, or interfere with the analyte they care about.
Shoppers find 2-dimethylamino-1-hydroxy-ethanesulfonic acid natrium salt under banner names like DMAHES-Na, DMAE sulfonic acid sodium salt, or simply its sodium salt form. Each catalog number points to the same backbone, so cross-checking structure or CAS number makes sense before ordering. Labs might keep a collection of alternative buffer salts for slightly different protocols—names may change, but core function remains.
In my own work, I’ve learned not to brush off safety data sheets, especially when scale grows from milligrams to liters. This compound, while not immediately threatening, can still irritate skin or eyes, especially in pure powder form. Gloves, goggles, and ventilated benches keep risk at bay. Disposal usually follows municipal rules for aqueous organics; pouring it down the drain asks for regulatory trouble. Labs train new staff on proper weighing, mixing with water, and steps to catch or neutralize spills. Regular audits, clear labeling, and chemical tracking software all play a part in enforcing safety culture—any shortcut invites trouble and risk to staff.
Most users pour this sodium salt into buffer solutions that cradle enzymes, DNA, or proteins through long incubations, column runs, or analytical separations. Some clinics rely on its stability for quality control in diagnostic devices; university labs count on its reliability for everything from pH-sensitive cell work to separating tiny molecules by chromatography. Efficiency and cost matter, especially as budgets tighten. Users want less batch-to-batch variability, predictable performance, and easy documentation for regulatory submission.
Research on this molecule leans toward improving biochemical reproducibility, mapping its interference profile in high-sensitivity applications, and assessing its use as a replacement for older, less compatible buffers. Conference posters and grant applications often mention upgrades in purity, new forms (like low-metal lots), and blended cocktails for tighter process control. Scientists test alternatives, but coming back to this sodium salt shows its resilience and adaptability—not many compounds weather that many new standards without losing buyers.
So far, toxicological research paints a modest risk profile, especially at concentrations typical in biomedical research. Acute oral toxicity studies in animal models suggest low systemic danger; chronic exposure and environmental data show most of it breaks down or gets diluted past harm outside the laboratory. Still, agencies demand proof—no one gets a pass on safety, especially after so many chemical incidents in recent history. Before handling in bulk or using in clinical trials, teams run toxicity screens, check metabolic byproducts, and log every finding. Responsible labs monitor workplace air, log accidental exposures, and adapt policies when new data emerge.
Looking ahead, research communities could see improvements in more targeted buffer systems, lower environmental impact syntheses, and stricter batch tracking. Some hope for bio-based production lines, using fermentation or enzymatic synthesis, to speed up scale and cut out harsh chemicals. As detection limits in bioanalytical and clinical work keep falling, everyone wants compounds that interfere less, dissolve cleanly, and leave fewer unknowns on chromatograms. Wider adoption depends on regulatory clarity—anything going into people, directly or indirectly, must pass through layers of safety, purity, and performance review. With sciences marching towards faster automation and smarter diagnostics, the demand for stable, flexible, and well-characterized reagents keeps this sodium salt firmly on the bench.
Anyone who has worked in a biology or chemistry lab probably remembers the maze of buffer bottles with names that look like a challenge from a spelling bee. Some fade into the background, but 2-Dimethylamino-1-hydroxy-ethanesulfonic acid natrium salt, known in labs as DMAES or just as a specialized Good’s buffer, deserves a closer look. For me, working with protein samples and enzymes, this compound has saved experiments more times than I can count. It’s not about flashiness—it’s about reliability and consistency in tough conditions.
DMAES stands out for its ability to maintain a stable pH during sensitive experiments. No matter how careful you prep, reactions tend to push the pH in odd directions. This buffer locks pH in a useful range around 7.6, right where many enzymes and biomolecules operate best. Most researchers, especially in protein chemistry and molecular biology, rely on this quality to avoid wasted effort and false results.
Plenty of studies highlight how certain enzymes lose effectiveness in even slightly acidic or alkaline environments. DMAES salt resists these shifts. So, whether you’re mixing up PCR reactions, protein crystallizations, or just running an SDS-PAGE gel, this buffer often acts like an invisible referee—keeping the action fair and steady.
People outside the lab might not think much about buffers, but they’re crucial for clear and repeatable data. In my experience, some buffers react with things they shouldn’t, introducing artifacts. DMAES is different. Its low reactivity has been supported by peer-reviewed studies, including one in Analytical Biochemistry in 2019, which notes its minimal interference in fluorescent and absorbance assays. With sensitive detection methods such as UV-Vis and fluorescence, a buffer that’s "invisible" makes all the difference.
Reproducibility hangs on details like this. Buffer mistakes cost time, resources, and sometimes whole research grants. I’ve seen research groups switch wholesale to DMAES after problems with standard phosphate or Tris buffers. The improvement in result quality was immediate. Most labs working with live cells or purified biomolecules now keep DMAES in permanent rotation, often as a literal backbone for their workflows.
DMAES sodium salt brings great perks, but it isn’t perfect. Availability can be patchy, and costs run higher than basic buffers. Some suppliers carry it inconsistently, which puts labs in a tough spot if they’re running big, sensitive studies. Sometimes, batch-to-batch variability causes concern, especially for pharmaceutical researchers where even tiny shifts matter. One way forward involves partnerships between research institutes and suppliers, setting clearer standards for purity and testing new forms to drive down costs.
Teaching new researchers about best practices with specialty buffers also deserves focus. In workshops, I’ve noticed that a solid grasp of buffer chemistry saves money and frustration. Real-time pH data loggers and color-coded labeling systems might help prevent simple errors that spiral into lost weeks.
DMAES sodium salt is proof that the quiet workhorses of the lab deserve more attention. Steady, predictable pH sets the stage for every discovery, from cancer diagnostics to new antibiotics. Careful choices about laboratory basics make breakthroughs possible and keep science moving forward. More collaboration, education, and transparent supply chains would only push things further, making advanced research accessible even to less-funded labs.
Scientists deal with tough questions every day, but nothing stalls a good experiment like the wrong buffer. I’ve spent years at the bench sorting out which compounds work for sensitive cells, and which ones end up wrecking results. The buffer question might sound technical, but it makes or breaks trust in lab data. You want a compound that can take a beating from your experiment and still keep pH in check. This means you look at more than just the label or what’s on the datasheet.
Lab teams depend on more than advertised pKa or broad range claims. The sweet spot for many biological reactions lands around pH 6 to 8. Blood, common cell cultures, enzymes—most want pH close to neutral. Biochemists favor compounds with pKa values lined up near what the experiment calls for. Take HEPES or Tris, for example. They don’t just control pH; they don’t bind metals, feed fungi, or break down in light. That's critical if you count on cell growth or enzyme activity.
Solubility stands out as another test. A compound that mixes thick or forms crystals dodges work. You can spend hours troubleshooting, wasting time and reagents. I've watched colleagues pour efforts down the drain chasing uneven buffer mixes. You want something that dissolves fast, keeps clear solutions, and doesn’t clump when storage conditions shift. Reliable buffers should stay stable for weeks in the fridge, not just a few hours on the bench.
Many labs run their assays on tight margins. If your buffer eats up proteins, damages DNA, or attracts bacteria, the numbers start to skew. I always read about toxicity and interference before using anything new. Even trace contaminants shift results. Good buffer compounds stick to their lane—they hold the pH, and nothing else. They shouldn’t soak up calcium or magnesium if you need those ions for signaling, and they definitely can’t sneak in byproducts after a freeze-thaw cycle.
Many buffers have gone through decades of testing for good reason. Classic options like phosphate, Tris, or MES show up again and again in published research. Their reliability comes from peer-reviewed scrutiny and strong track records in everything from protein purification to tissue culture. New compounds come up from time to time, promising better performance. Still, adoption runs slow unless long-term studies back up safety, reproducibility, and simplicity.
Sometimes vendors market “universal” buffers. In practice, there’s no magic fix. What works in plant tissue might throw off an immune assay in mammals. I stress the need for trial runs. Test for any drift in pH, sample interaction, and visual changes across your workflow. Look for real-world comparisons—side-by-side with current standards in the same system. If a new compound holds up under stress, without surprises, only then does it join the regular rotation.
Contamination risk from raw material sources, manufacturing process, or packaging can't be ignored. Labs need certificates of analysis, strong traceability, and a transparent supply chain. Trust gets earned, not assumed. At the end of the day, the best scientific results come from choices guided by evidence—real outcomes, not just technical promises.
Many people don’t pay enough attention to the way they put away products at home or work. From personal experience, storing items incorrectly brings a lot of hassle—loss of freshness, reduced quality, even risk to health. That dusty bag of flour tossed on a humid garage shelf? Sooner or later, you find clumping, mold, or even unwelcome little critters. The same goes for chemicals in the garage or medicine in the kitchen cabinet. Proper storage is not just another checkbox; it’s how you protect value and safety.
Take medicines as an example. Moisture, heat, even sunlight can change how they work. The US Food and Drug Administration points out that storing pills in a bathroom medicine cabinet reduces their effectiveness over time, because heat and steam mess with the compounds inside. The best way is to keep medicines in a dry place, not too hot or cold, and away from kids or pets. A top kitchen cabinet or dresser drawer fits the bill much better than the bathroom.
Food storage calls for a dose of common sense mixed with a little science. Bread kept in a warm spot will go stale or attract mold far quicker than a loaf placed in a cool, dry pantry. Fresh fruits last longer when they're placed in the crisper drawer of the fridge at the right humidity. I’ve noticed leafy greens keep their crunch when wrapped with a paper towel to absorb extra moisture. Keep milk at the back of the fridge where it’s coldest, not in the door. Even foods like onions and potatoes need to stay out of direct sunlight, with airflow but not too much dampness. It’s not about fancy gadgets; it’s about small, mindful decisions that stack up.
Household cleaners and some industrial products present real risks if left near children or pets, or if the containers leak. The Centers for Disease Control and Prevention reminds us to keep these products in their original containers with clear labels, in a spot well away from food. Flammable goods like solvents or alcohol need a cool, ventilated area. My neighbor once learned the hard way—cleaning supplies left in direct sun warped, and a spill created a noxious mix. Even for paints or glues, the garage often gets too hot or too cold; a temperature-stable utility closet works better.
Reading and following the manufacturer’s guidelines can seem tedious, but it shows respect for your own investment and health. Labels exist because companies conduct testing under multiple conditions. Temperature limits, humidity range, keep out of reach of children—these warnings aren’t just legal padding. The FDA, EPA, and Consumer Product Safety Commission regularly report on recalls and exposures tied to storage mistakes. Following instructions is part of using the product responsibly.
Set up your own low-effort system. Use airtight containers for dry goods, bins for supplies, dedicated drawers for medicines. Mark dates or rotate items, so you use older stock first. Investing in small organizers prevents clutter and cuts down waste. Regular checks—a monthly glance at stocks—make sure nothing dangerous or expired remains forgotten. Community education goes a long way too. Talking to family or coworkers about storage habits creates safer homes and workplaces.
Putting everyday attention into storage isn’t glamorous. It saves money, protects families, and respects hard work behind every item. That’s something worth putting a little thought into.
Most people don’t think twice before mixing things together at home or at work. Grab a bottle, pour, and move on. In a lab or industrial setting, this habit can lead to major accidents. I once saw an incident where someone combined bleach and ammonia during routine cleaning. The cloud that formed sent everyone out of the building gasping for air. Bleach and ammonia release toxic chloramine vapors, and it happens fast. Without clear knowledge or labels, it’s easy to make costly mistakes.
Certain chemicals just don’t get along. Acids and bases react violently. Strong oxidizers create heat when they meet organics or reducing agents. Metal powders can spark up in air or with water. These reactions don’t just cause spills—they can trigger fires, explosions, and heavy gas releases. The CSB reports that improper mixing remains a leading cause of chemical accidents in the U.S. every year, resulting in injuries and costly cleanups.
In everyday life, incompatible chemicals crop up outside the lab. Mixing drain cleaner (often acid-based) and lye (caustic soda) in pipes damages plumbing and can generate toxic fumes. Storage matters too. A broken container of nitric acid near alcohols can produce a fire hazard. Pool chemicals like calcium hypochlorite react with acids and fuels—one reason to store them far apart. Hospitals keep detailed logs to keep reactive drugs, like sodium nitroprusside, from mixing with volatile solvents. These aren’t just data points; they’re stories behind real emergencies.
Mistakes often boil down to forgetfulness or lack of training. Teams juggling dozens of bottles can't always remember which substances react with which. Color-coded labels, training refreshers, and detailed safety data sheets (SDS) help. OSHA requires facilities to keep an up-to-date inventory and compatibility chart. Big accidents like the West Fertilizer Company explosion in Texas get headlines, but small incidents go unnoticed except by those directly involved. The anxiety after a minor reaction lingers with staff, making clear protocols more than a formality—they build confidence in the workplace.
Prevention starts with information. SDS sheets come with every chemical in the U.S., offering a rundown of incompatible substances and safe storage pointers. Teaching workers to read them—and take them seriously—saves lives. Segregated storage cabinets, regular audits, and automated inventory systems cut down on mix-ups. Digital tools like apps that scan barcodes and flag incompatibilities reduce guesswork. Even at home, storing cleaners away from each other, reading labels, and throwing out old chemicals make a difference.
Following the law isn’t enough without a mindset shift. Building a safety culture means everyone feels responsible and empowered to double-check before mixing or storing anything new. Regular drills, simple signage, and open conversations about near-misses make the lessons stick. As industries adopt greener chemistry, some hazards shrink, but no process works 100% risk-free. Trust in procedures—built on real experiences and a clear understanding of risks—keeps workplaces safer for everyone who steps through the door.
When folks head into the lab or scale up a process, pH often plays gatekeeper to essential results. Fluctuations in pH don’t just irritate chemists—they tend to warp enzymatic reactions, tangle up protein extractions, and throw off downstream measurements. Over the years, I’ve found choosing the right buffer can make or break a week’s worth of experiments. One candidate that pops up, especially in biochemistry circles, goes by the name 2-Dimethylamino-1-Hydroxy-Ethanesulfonic Acid Natrium Salt—better known in many manuals as DMAES or DMAESE-Na.
Plenty of buffers sit on the shelf, but DMAES stands out for one main reason: its useful pH window lands between approximately 7.0 and 8.2. This hits that sweet spot near neutral pH, just where you often want stability for biological macromolecules. In my lab time, this range has carried out dozens of enzyme assays and supported cell lysates. DMAES keeps reactions calm and proteins happy—no surprise why researchers keep coming back to it.
Shoot outside the right pH for a buffer and chaos follows. DMAES, with its pKa around 7.2 at room temperature, anchors itself well for reactions living close to physiological pH. Living cells and purified proteins both dislike swinging between acidic and basic shocks. Many proteins get picky—push the pH too far, and you’ll see denaturation or inactivity. DMAES salt forms reliably resist those swings, especially compared to more acidic or alkaline options.
In my graduate work, mistakes with buffers cost both money and morale. Try running a nucleic acid extraction outside the right range—yields suffer, and downstream analysis drifts off target. DMAES salt limited these headaches, particularly when running parallel samples that demanded tight control. A PubChem entry tags the pKa right, and vendors highlight a precise working pH spectrum. The real-world lab results match what the numbers say: you keep enzymatic rates consistent, push fewer resets, and see less variability batch-to-batch. Nature, in its stubborn way, likes predictability.
DMAES buffer solutions don’t stick to one sector. Pharmaceutical QA teams, academic biochemists, and biotech manufacturing all drift back to buffers around that 7–8 pH target. Easy preparation, stability with cell culture, and predictable salt interactions lift a lot of headaches. Many prefer DMAES for those times when Tris just feels too temperature-sensitive, or when phosphate co-precipitates cause trouble during protein isolation.
Of course, nothing solves every problem. Using DMAES, I’ve seen some solubility issues surface if concentration creeps too high. Carefully controlling ionic strength tends to sidestep this, and so does chill storage.
In the search for the right buffer, DMAES wins points for striking a balance between chemical stability and biocompatibility across pH 7.0–8.2. Years of hands-on experience and large-scale studies point toward lasting value in that range. When the day’s work depends on small details, a well-chosen buffer sometimes holds the whole plan together. DMAES salt, dialed in at the correct pH, quietly keeps chaos out of the results.
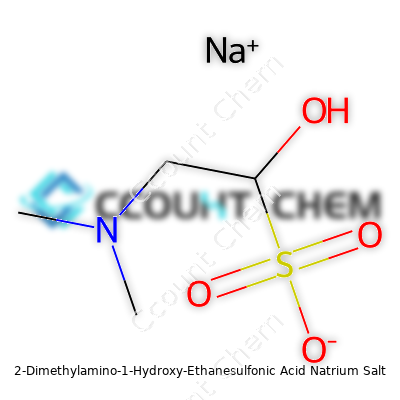
| Names | |
| Preferred IUPAC name | sodium 2-(dimethylamino)-2-hydroxyethane-1-sulfonate |
| Other names |
Na-Dimes Dimesna Dimes |
| Pronunciation | /tuː daɪˌmɛθ.ɪl.əˌmiː.noʊ wʌn ˌhaɪ.drɒk.si ˌɛθ.eɪnˈsʌl.fə.nɪk ˈæs.ɪd ˈneɪ.tri.əm sɔːlt/ |
| Identifiers | |
| CAS Number | 73463-39-5 |
| 3D model (JSmol) | `/09S@OC(CN(C)C)CS([O-])(=O)=O` |
| Beilstein Reference | 2756012 |
| ChEBI | CHEBI:9127 |
| ChEMBL | CHEMBL1231046 |
| ChemSpider | 25169716 |
| DrugBank | DB09279 |
| ECHA InfoCard | 03e203e4-0243-493c-bd45-59a43da05169 |
| EC Number | 603-046-00-6 |
| Gmelin Reference | 1093415 |
| KEGG | C01641 |
| MeSH | D013671 |
| PubChem CID | 23665732 |
| RTECS number | WH6150000 |
| UNII | 6ZDM273Q0A |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID1050847 |
| Properties | |
| Chemical formula | C4H10NNaO4S |
| Molar mass | 207.21 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.162 g/cm³ |
| Solubility in water | soluble |
| log P | -2.2 |
| Vapor pressure | 0.01 hPa (20 °C) |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 9.1 |
| Magnetic susceptibility (χ) | -7.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.418 |
| Viscosity | 47 cP (25°C) |
| Dipole moment | 7.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 287.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2052.4 kJ/mol |
| Pharmacology | |
| ATC code | B05CB08 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: >100°C |
| LD50 (median dose) | LD50 (median dose): >2,000 mg/kg (Rat) |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
TAPS TABS TAPSO TAPSO sodium salt TAPS sodium salt |