2-(Cyclohexylamino)ethanesulphonic acid, known in the lab by the catchy acronym CHES, didn’t land in chemistry textbooks overnight. A wave of interest in biological buffers during the late 1960s and early 1970s nudged researchers to look for alternatives to messy, unreliable buffer salts. Norman Good and his team fired up this drive by mapping out criteria for ideal biochemical buffers. They ended up putting compounds like CHES on the map, not just for their stability but for their quiet contribution in routine lab work. The demand for precise control over pH, especially in experiments leaning toward alkaline conditions, kept CHES in the game. Its name echoes through countless research papers now, mostly because scientists grew tired of unpredictable results from clunky early buffers.
Walk into any life sciences supply store, and CHES powder sits among the essentials on the shelf. There's a reason why: labs need stable pH control, and CHES delivers. Commonly supplied as a white crystalline powder or sometimes a granular solid, its ability to maintain a steady pH between about 8.6 and 10.0 makes it regularly chosen for protein purification and enzymatic assays. The presence of the cyclohexyl group tames extreme chemical behavior, giving CHES a long shelf life and exceptional solubility compared to more basic buffer choices of decades past. Chemists don’t waste effort on complicated preparation—solutions come together with just deionized water, and it doesn’t degrade in glassware, meaning no one has to toss a batch due to odd results.
Dumping a scoop of CHES out of a bottle, the physical attributes come right into focus. It’s a clean, odorless solid that doesn’t clump or turn sticky in humid climates. Molecular weight clocks in at 207.3 g/mol. It melts above 280°C, which limits accidental decomposition during standard lab work. With a pKa of about 9.3 at 25°C, it cushions processed solutions from sudden pH swings near that value. Water swallows it up fast: up to around 50 g will dissolve per liter at room temperature, which beats a lot of its pH-matching cousins. Lugging buckets of electrolyte won’t get you the same clarity—so CHES stays on shopping lists year after year.
Labels on commercially available CHES don’t hold back on detail: CAS number 103-47-9, molecular formula C8H17NO3S. Purity hovers at or above 99%, since contamination sours delicate biochemical tests. Makers often guarantee low levels of heavy metals and other quirks that would spoil high-throughput experiments. Many suppliers include batch-specific pH performance data, so researchers can spot even tiny shifts. Each bottle lists safe handling instructions, given that safety eyes glass this stuff as an irritant. Long shelf life comes from proper sealing, and labels remind users about dryness and storage away from reactive agents.
Back behind the scenes, making CHES taps into simple chemistry. Cyclohexylamine jumps into a reaction with 2-chloroethanesulphonic acid, and a straightforward substitution pops the chloride out, letting cyclohexylamine cling to the ethanesulphonic framework. The resulting product gives up its impurities in a few rounds of crystallization. Lots of chemical suppliers rely on this basic protocol, so batches look similar whether made in a commercial-scale reactor or small-batch instruments for research use. This approach keeps costs fair while supplying plenty for the research sector's appetite.
CHES, built as a sulfonic acid, shows off its chemical resilience day in and day out. In water, the acid group ionizes to steady the pH; in organic solvents, only the most aggressive reactants manage to take a swing at the cyclohexylamino backbone. Want to tweak the buffering range? Chemists sometimes fiddle with substitutions on the ring. Yet, most labs just lean on CHES’s established buffering power and leave it unchanged. With a stable sulfonic group, hardly any oxidation-prone functional handles exist. This dead-simple reliability lets it slip quietly into countless reaction protocols without drawing attention or hassle.
Tracking CHES through the literature, you’ll spot names that vary by source and region. The phrase “2-(Cyclohexylamino)ethanesulphonic acid” spells out the structure, but a stack of suppliers also print labels as “CHES buffer,” “Cyclohexylaminoethanesulfonic acid” or “N-Cyclohexyl-2-aminoethanesulphonic acid.” Students and professionals alike settle on the four-letter abbreviated version, which often saves a headache trying to fit long names onto data entry forms or shelf tags. The world’s research community shares a single CAS number (103-47-9) to avoid type-o confusion.
There’s more to CHES than just a bland white powder. Handle it without gloves, and a skin tingle follows, and dusty air brings coughs if you forget a mask. Most lab techs stick with standard goggles and nitrile gloves, and work in spots with plenty of airflow. No one likes trips to the eyewash station, so spills get cleaned up immediately with damp cloths. Suppliers include hazard statements, even though CHES ranks low in acute toxicity. Labs handling industrial volumes pack dust masks or respirators and dump sweepings in tightly sealed hazardous waste bins. Emergency procedures look routine, focusing more on practical steps than elaborate documentation.
Ask around in the protein purification field and CHES gets mentioned in the same breath as trusted buffers like HEPES and Tris. Plenty of enzyme assays count on CHES pushing pH at the higher end, so key reactions can play out without pH drift. Diagnostic kits lean on its solid stability, while instrument manufacturers recommend it for reducing background noise in sensitive analytical assays. Large-scale biotech plants rely on bulk shipments as they process fermentations, optimize enzyme activity, or prep protein solutions for downstream processes. Bacterial cultures run better, using this buffer compared to alternatives that fade fast near alkaline pH. Its adaptability keeps it circulating from basic research to applied industrial workflows year after year and generation after generation.
Research teams spin up new buffer formulations year in and year out, yet CHES has kept researchers’ trust. Scientists testing next-gen enzymes return to CHES whenever they seek minimal autofluorescence and consistent pH maintenance. Biochemical research on membrane proteins, notoriously tricky to stabilize, favors its low ionization interference. Some development teams look at minor modifications, exploring whether tweaks to the cyclohexyl group or the amine functionality might extend workable pH ranges without betraying chemical stability. Analytical chemists have reported CHES serving as a background electrolyte in capillary electrophoresis and other high-precision analytical techniques. Studies testing compatibility with modern gene expression workflows continue to pile up, driven by the need for ever more reliable laboratory workflows.
Despite decades of routine use, thorough toxicity assessments form part of any reputable buffer chemical’s record. Animal tests indicate low acute toxicity, yet CHES, like many sulfonic acids, calls for strict handling to prevent accidental ingestion or inhalation. Eye and skin irritation stays on the radar, especially during large-scale production or frequent solution mixing. Long-term exposure considerations hinge on minimizing airborne dust and direct contact. Standard practice means keeping CHES out of aquatic environments—ecotoxicological data suggest sulfonates resist breakdown, raising concerns about persistence. Risk assessments by regulators nudge handlers toward closed systems wherever possible. In academic and production settings, labs train staff for proper disposal so no unnecessary health hazards or environmental fallout develops downstream.
Chemistry never stands still. Even with many reliable years behind it, there’s no guarantee CHES will hold top billing forever. Emerging synthetic biology routines might stretch the boundaries for pH sensitivity, spurring chemists to try new substitutions and responsive systems. With green chemistry on the rise, researchers may soon lean harder into developing biodegradable alternatives, or at least formulas that don’t persist in waterways. As automation moves into more labs, CHES’s trusted pH control can help machines run research more efficiently and with fewer mishaps. New packaging approaches, like pre-measured single-use pouches, may draw in next-generation users keen to boost throughput and reduce handling risks. As tech evolves, CHES’s next chapter will likely see tailored roles in precision manufacturing, advanced diagnostics, and the ever-growing landscape of computationally designed biological reactions.
Across the world of laboratory science, some substances don’t get much spotlight, yet everything would grind to a halt without them. 2-(Cyclohexylamino)ethanesulphonic acid, most folks know it as CHES, fits that category. In the many research labs I’ve spent time in, CHES frequently turned up on the shelf, not as some rare reagent, but as a reliable buffer that made just about every experiment run a bit more smoothly.
A buffer does the unglamorous job of keeping the pH steady in solutions during experiments. That doesn’t sound like a big deal, but molecules can get moody. Change the pH too much, and they refuse to behave. Without a dependable buffer, results run off the rails, wasting hours of work. CHES stands out because it keeps things even in a pH range from about 8.6 to 10. That’s a sweet spot for studying enzymes and proteins that thrive in slightly basic environments. CHES kept things steady through countless protein analysis runs, letting me focus on the details instead of troubleshooting unstable conditions.
The reason labs value CHES goes way beyond convenience. Research isn’t just about moving colored liquids between little tubes. Scientists build everything they learn on repeatable, reliable experiments. Hospitals and clean water technologies draw from these discoveries. As a buffer, CHES supports biochemical research around enzymes, which play roles in everything from diagnosing diseases to developing medications. Stable buffers lead directly to more trustworthy data, and ultimately to better healthcare and cleaner industries. If you care about faster advancements in medicine or environmental testing, you probably have buffers like CHES to thank, even if you’ve never heard its name.
I remember the first safety briefing in my undergraduate lab. The instructor picked up a bottle of buffer – not CHES, but similar. “Don’t be careless just because these aren’t acids or solvents,” she said. Sure, CHES isn’t particularly volatile or toxic compared to many other chemicals. Still, every responsible lab worker keeps gloves handy, uses eye protection, and works in ventilated spaces. Chemical manufacturers supply data sheets outlining safety measures for a reason. It’s not about anxiety or paranoia, it’s about respecting the few grams of powder that make or break expensive research efforts.
Purity can trip up even experienced researchers. Off-brand buffers or poorly stored containers ruin entire experiments. Once, a contaminated buffer ruined weeks of careful enzyme study, driving home the lesson that sourcing matters. Trustworthy suppliers, batch-testing, and proper labeling save money and time in the long run. Good scientists watch backups closely and don’t cut corners just to save a few bucks on chemicals.
Science keeps moving. Digital pH meters and automated mixing systems have reduced manual errors. Still, everything depends on the chemistry of the buffer. New formulations now aim to reduce waste and improve biodegradability. There’s always scrutiny about what enters our environment when labs rinse out flasks. By choosing the right buffer, and knowing how to dispose of it correctly, labs shrink their chemical footprint. Regulators and manufacturers keep tweaking instructions, making it easier for students and seasoned pros alike to handle CHES and its cousins responsibly.
CHES will never headline the news, but for anyone building experiments from the ground up, it’s a constant companion. Easy to overlook, but crucial for steady hands and sharp science.
Nobody wants a ruined batch of 2-(Cyclohexylamino)ethanesulphonic acid when precision matters most. It shows up in many labs as a useful buffer, especially in biology, so a little care can spell the difference between smooth experiments and confusing results. Many researchers, myself included, have lost days chasing phantom errors that boiled down to unstable reagents or poor storage. Over time, I’ve learned the conditions you keep your chemicals in can tell as much of the story as the experiment itself.
Storing this compound anywhere humid or hot risks inviting trouble. Best practice, drawn from both manufacturer advice and real lab stories, calls for a cool, dry place. Room temperature works for short stretches, but for anything longer, dropping it into a fridge (2–8°C) takes away the guessing. Fluctuating heat and sunlight speed up decomposition, and that can mess with pH and consistency in sensitive tests.
Not every lab labels their shelves by temperature suitability, but storing reagents alongside similar buffer chemicals, away from heat sources and direct sunbeams, stands as the surest path to keeping everything predictable. In one stint at an environmental lab, we tracked storage temp with simple thermometers—no mystery, just steady readings, and no failed controls.
Hygroscopic powders soak up moisture like a sponge. Even if 2-(Cyclohexylamino)ethanesulphonic acid isn’t the worst offender, it's worth storing it with a tight cap and using desiccant packs in the container. I remember opening a jar only to find a stubborn clump—it still dissolved, but it left questions about purity. Fresh chemicals go straight from supplier bottle to sealed secondary containers, with silica gel nearby.
Wet environments—say, near a busy sink or under a dripping air conditioner—only increase the odds of contamination. Clean, dry scoopulas and spoons prevent introducing water. Some places train new staff by having them weigh empty containers to check for moisture before and after use. These small habits stick with you, and they preserve more than time. They defend results.
The acid stays stable if it sits by itself, but things go wrong fast if it meets strong oxidizers, acids, or bases. Separate storage shelves, clear labeling, and tight lids make life easier. In a mixed-use teaching lab, one shelf collapsed, mixing ordinary buffers with oxidizing tablets. We learned quickly—one cabinet just for buffers, locked during off-hours, saved weeks of grief later on.
Even stable reagents have a shelf life. Every bottle in my old university lab carried a bold label: purchase date, open date, and initials. Replacing rarely-used acid each year trimmed supply waste and kept reactions on track. It doesn’t just help in audits; it’s self-defense against nagging uncertainty. If something grows, smells off, or changes color, it moves to the chemical waste bin.
Clean and dry hands win half the battle. Routine checks, clear rules, and respect for chemicals cut costs and stress. Talk to the people who do most of the buffer prep—more than manuals, their workarounds stop waste and confusion before it begins. Good storage gives confidence, saves experiments, and takes nothing for granted. Chemistry stays simple when the basics get real attention.
2-(Cyclohexylamino)ethanesulphonic acid, better known in science labs as CHES, gets used a lot in biology and chemistry. Most folks working with proteins or enzymes recognize it as a buffer—a tool for keeping pH levels steady so experiments don’t spiral out of control. Plenty of research papers and lab manuals list CHES right next to other familiar buffers, so it’s easy to assume it’s got a low risk profile. But like a lot of chemicals, its safety record depends mainly on how it’s used and the amount handled.
I remember my first year in a molecular lab. A senior technician told me, “All buffers are safe until you get careless, and then none of them are.” The truth is, most sources—including Sigma-Aldrich and Carolina Biological—label CHES as an irritant. Touching it can make your skin or eyes sting and go red. Accidentally inhaling its dust, or breathing it in every day for months, can irritate airways or cause coughing. Most people don’t eat chemicals in the lab, but accidental ingestion could upset your stomach, just like other mild irritants.
There haven't been publicized disasters or scares linked to CHES, unlike what you see with mercury or strong acids. The US National Library of Medicine doesn’t list it as a carcinogen or as something that builds up in your body or in wildlife. The European Chemicals Agency (ECHA) marks it as “hazardous for skin and eyes, but not mutagenic or toxic by everyday exposure standards.” So no, it’s not among the most toxic lab chemicals, but that doesn’t make it harmless. It’s irritation you watch out for, not life-threatening poisoning.
Lab safety guidelines shape how CHES gets stored and handled. The Global Harmonized System for chemical safety gives it a warning sign for skin and eye reaction, but not the flaming-red “danger” for explosive or highly poisonous substances. Many university safety data sheets echo that: basic gloves and goggles, plus prompt washing if you get splashed, are enough to keep routine use safe. This aligns with the experience of the vast majority of researchers—thousands handle it daily without incident so long as protective equipment goes on and spills get cleaned right away.
Carelessness or unclear protocols invite accidents, even with less infamous chemicals like CHES. Some lab accidents catch people off-guard, like rinsing ungloved hands after handling powder, then rubbing their eyes. Even mild eye exposure stings and can disrupt lab work for hours. No major animal or human toxicity studies have flagged severe risks, but repeated exposure over years may still have unknown health impacts. That’s why it helps to train new lab workers to expect mild irritation and to respect every bottle of chemical—buffers included.
I stick to the basics: never eat or drink in the lab, never touch my face before washing, and always recap bottles after pouring out what I need. Clean habits, honest reporting of accidents, and good communication make a world of difference. Even though CHES doesn’t come with scary warnings, treating every new chemical with a dash of healthy respect looks out for both you and your labmates.
One solution for any risk is never assuming any chemical is "too safe." Routine training, gentle reminders, and regular labeling checks stop the vast majority of mishaps. If you spill CHES, rinse with lots of water, dry the area, and tell your supervisor—no shame in speaking up. Simple, practical habits keep hazards from piling up, even with substances as widely used as CHES.
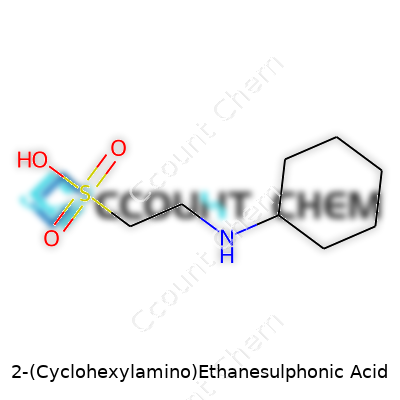
Plenty of lab workers recognize 2-(Cyclohexylamino)Ethanesulphonic Acid, but many just call it CHES. Looking at the formula, you’ll find C8H17NO3S staring back at you. The molecular weight lands at about 207.29 g/mol. This compound may seem like just another entry in a chemical catalog, but it shows up all the time in biochemistry labs. The story really starts with how handy CHES gets when researchers chase specific pH targets in their solutions.
Years ago, I spent long afternoons troubleshooting enzyme reactions that kept crashing without clear reasons. It didn’t take long to realize that the problem came down to pH changes outpacing expectations. That’s the daily grind in the lab. If a buffer collapses, the entire experiment follows suit. CHES, with its structure and sulfonic acid group, steps up to the plate between pH 8.6 and 10. This isn’t just another salt dumped into a flask—it holds pH steady in a stubborn range where plenty of other buffers wobble.
Other buffers, like Tris or HEPES, tend to dip out at higher pH. CHES covers the gap. This isn’t just chemistry for chemistry’s sake. Medical diagnostics, protein purification, and research into enzymes all lean on precise pH levels to get honest results. Without buffers like CHES, finding reliable numbers turns into a guessing game.
Labs can easily buy CHES off-the-shelf, but purity makes or breaks research. Low-grade batches bring along unwanted leftovers that mess with sensitive reactions. Whenever budgets get tight, it’s tempting to squeeze savings from raw material quality. In my own experience, impure reagents can burn days of effort, leaving results that have to be tossed out. Investing in reputable suppliers keeps the focus off chasing chemical ghosts and on getting valuable data.
Exposure rarely causes serious harm, but nobody walks through the lab pretending every powder is sugar. Mishandling CHES dust can irritate eyes or skin. Good lab routines always kick in—use gloves, goggles, and a properly ventilated area. Even confident chemists run through safety data sheets and take stock of storage needs, knowing that a single lapse can cause headaches for the whole team. Clean and labeled containers, paired with regular checks of expiration dates, save trouble before it starts.
Getting rid of used buffer solutions calls for thoughtful planning. Pouring sulfonic acid derivatives down the drain doesn't cut it. Local regulations demand proper waste handling. Many labs gather acidic or basic waste for dedicated disposal services. When teams stick to the rules, water systems and soil stay out of harm’s way. Focusing on responsible chemical use sets positive standards for all who come after.
CHES, with its clear-cut formula and distinct properties, has a big footprint in research settings. Each lab decision—pick the right buffer, stick to safe handling, dispose of waste the right way—decides how well teams can trust their results. Science grows stronger when these day-to-day choices become habits.
Lab benches can turn messy in a hurry, but it pays to know your chemicals. 2-(Cyclohexylamino)Ethanesulphonic Acid, often called CHES, stands out in biochemistry labs, thanks to its stable nature and consistent pH buffering near 9.5. Scientists lean on it while running protein studies, enzyme reactions, and other setups that can't stand unstable pH. Powdered CHES looks pretty harmless—a pile of white or off-white granules. Before jumping in, double-check the label and storage, as moisture absorbs quickly and can mess up weigh-outs.
Some folks think grabbing a lab coat and gloves covers all the bases, but even so-called mild buffers like CHES ask for respect. The powder has a knack for floating up your nose, so a face mask keeps breathing easy. Working in a clean, well-ventilated area stops spills from turning into headaches later. I always tell lab mates to keep a bottle of distilled water close, just in case. If a splash hits your skin, rinse it right away. Clean habits save time and headaches.
Pull up the chemical’s datasheet for solubility data. CHES dissolves well in water at room temperature, but fireworks start if you try to hot-dissolve too much at once. I measure the powder on a calibrated balance, careful to tap excess off the spatula. For a standard 0.1 M CHES buffer, weigh out the grams to fit your desired volume, usually using the formula: Moles = Molarity × Volume. The purity on the label matters—some batches run higher than 98%, but always check for additives or moisture content.
Pour the calculated powder into a beaker already holding about 80% of your desired final volume of deionized water. Stir gently with a magnetic stir bar. No need for aggressive mixing since CHES dissolves better with patience. If you must adjust pH, most folks use sodium hydroxide, adding drops slowly while monitoring with a calibrated pH meter. Resist the urge to dump in all at once—the pH jump can be sudden. After pH levels off around your target (like 9.5), top up with water to reach the exact final volume.
Sometimes, the solution seems milky or won’t clear, even after 10 minutes. Some blame it on poor water or contaminated glassware. Give your glassware a good wash with detergent and double-rinse with deionized water. If the room feels chilly, moving the beaker next to a gentle heat source (not a burner) often helps finish dissolving stubborn grains. Never heat with a flame, as CHES doesn’t like direct heat and fumes shouldn’t drift around the lab.
Once dissolved, use a clean storage bottle with a secure cap. Label everything with concentration, date, and your initials. CHES solutions keep for months in the fridge, staying clear and stable if made with deionized water and kept tightly capped. If you notice crystals or cloudiness later, discard the solution and start fresh. Stale or contaminated buffers throw off experiments and waste days of work. No trick beats careful mixing and clean habits.
Plenty of students trip up by adding acid or base too quickly, overshooting the target pH. Any strong acid or base works for pH adjustment, but the gentle touch wins every time. If you overshoot, don’t panic. Record the mistake, correct with the opposite, and document how much extra you added. Accurate logs mean you won’t repeat the mistake. Aim for honesty over impressing the next person who looks at your notebook.
| Names | |
| Preferred IUPAC name | 2-(Cyclohexylamino)ethane-1-sulfonic acid |
| Other names |
CHES N-Cyclohexyl-2-aminoethanesulfonic acid |
| Pronunciation | /tuː ˈsaɪkloʊˌhɛksɪl əˈmiːnoʊ ˌɛθeɪnˈsʌlfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 103404-87-1 |
| Beilstein Reference | Beilstein 3567312 |
| ChEBI | CHEBI:39050 |
| ChEMBL | CHEMBL113483 |
| ChemSpider | 87628 |
| DrugBank | DB03702 |
| ECHA InfoCard | 100.118.318 |
| EC Number | 103404-87-1 |
| Gmelin Reference | 82190 |
| KEGG | C01651 |
| MeSH | D03.633.100.221.173.190 |
| PubChem CID | 20568 |
| RTECS number | GV4375000 |
| UNII | GSK2X0V977 |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C8H17NO3S |
| Molar mass | 207.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.168 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -3.4 |
| Acidity (pKa) | 9.5 |
| Basicity (pKb) | 9.55 |
| Magnetic susceptibility (χ) | -5.7×10^-6 cm³/mol |
| Refractive index (nD) | 1.546 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 314.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -577.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4068 kJ/mol |
| Hazards | |
| Main hazards | H319: Causes serious eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 158 °C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 (oral, rat) > 8,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 8,000 mg/kg |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
CHES MES HEPES TES BES |