Chemicals like 2-Cyclohexylamino Ethanesulfonic Acid didn’t sprout up overnight. Labs built up knowledge layer by layer. Chain reactions of discovery often start with researchers wrestling with larger molecules, building buffers for reactions or chasing the puzzle of pH control. In the 1960s, as biochemical research spread across new cell-based frontiers, scientists needed buffers that worked at physiological pH values, weren’t toxic, and didn’t bind metals too tightly. Good’s Buffers—named after Norman Good—set the standard in bio-friendly chemistry. 2-Cyclohexylamino Ethanesulfonic Acid landed in this new buffer class, bridging gaps left by earlier substances that leached metals or failed to maintain a stable pH. This journey always feels personal to anyone who’s ever struggled with old-school buffer solutions, trying to keep cells alive in the dish, only to watch everything spiral out of control due to the wrong chemistry.
Nowadays, researchers running protein purification or running gels often reach for this acid as a staple buffer. Its use isn’t fashionable—it’s practical. In the lab, it usually comes as a white to off-white solid powder, punching well above its weight in usefulness. Whenever I handled it, the packaging always shouted the importance of dry storage and temperature control, showing how companies take stability seriously before it ever touches a pipette or a beaker. This acid sees wide distribution from chemical brands big and small, usually kept on the shelf next to its sibling buffers that fill in a different spot along the pH spectrum.
2-Cyclohexylamino Ethanesulfonic Acid has its own quirks. It has a molecular formula of C8H17NO3S and a molar mass near 207.3 g/mol. At room temperature it keeps its powder or crystalline form, dissolving easily in water but staying stubborn with less polar solvents. Its pKa sits around 9.5 at 25°C, so the buffer targets that basic pH range which fits nicely for proteins that shy away from acidic baths. Its solid stability helps with shipping and long-term storage but, as someone who's seen humidity ruin a good bottle, I’ve learned firsthand that dryness matters. This compound holds up in clear or slightly hazy solutions, a visual cue anyone can spot before running expensive experiments.
Labels do more than satisfy the auditor. Vendors stamp out purity grades, batch numbers, and trace metal content, because a speck of iron ruins an enzyme test or cell study. And every package includes shelf life, hazard info, and storage instructions. In regulated industries, it's common to get a stack of certificates outlining exact limits for contaminants, heavy metals, and other traces. Quality control depends on robust paperwork as much as on a sealed bag, leaving little room for shortcuts.
Synthesizing 2-Cyclohexylamino Ethanesulfonic Acid relies on bringing together cyclohexylamine and ethanesulfonic acid derivatives, usually through well-tuned condensation or substitution. Lab-scale prep combines reactants under controlled temperature, sometimes using acid chlorides or sulfonation as a route, and often finishing with careful purification—recrystallization, filtration, and drying. Good lab technique counts here: impurities, unreacted starting material, or even a careless wash can spoil the purity. On an industrial scale, automated systems take over, but bench-scale approaches underpin every good production run.
The compound takes part in limited side chemistry under mild conditions, keeping stable in most aqueous reactions. Still, strong acids or oxidizers can break it down, and some researchers have tweaked the structure at the amino group or sulfonic acid end to shift pKa or solubility. Rarely, derivatizations help tune the buffer for unique analytical uses, such as pairing with fluorescent tags, but most labs stick to the original recipe. People occasionally ask about metal chelation, but this acid generally leaves metal ions alone, unlike more complicated buffer systems.
Researchers and catalogues rarely stick to one name. Beyond its IUPAC label, it goes by CHES, N-Cyclohexyl-2-aminoethanesulfonic acid, and CAS number 103-47-9. Suppliers sometimes shorten or stylize names, but most people fall back on “CHES” for clarity. Anyone shopping for it in a lab supply catalog soon learns to keep an eye on synonyms to avoid ordering the wrong buffer salt or compound grade.
Safety chemicals always get a spot on the lab whiteboard. Contact with the eyes or skin brings irritation, and inhaling the dust is a no-go. In practice, gloves and goggles stop most accidents, a must for labs with strict standard operating procedures. Disposal calls for compliance with local regulations, since you don’t want this stuff in the water supply or trash. Experienced lab managers train new staff using real-life incident reports, and most modern vendors include SDS downloads with every purchase or shipment. Companies have raised the bar on traceability and emergency response just to keep pace with global safety demands.
CHES sees the widest use in life science and biochemical research. Protein chemists count on it for electrophoresis, enzyme assays, and column chromatography in the 8.6 to 10.0 pH range. During my grad school exam prep, I watched the buffer keep recombinant proteins stable through purification, saving days of work compared to trial-and-error pH adjustments. CHES crops up in chemical synthesis and water quality labs as well, whenever tight pH control is vital and amine reactivity must stay moderate. Quality assurance labs, especially in the pharmaceutical sector, often rely on those pH values for regulated tests. Its low metal binding finds use in microscope imaging or crystallography, where stray metals destroy fine-tuned results.
The journey of CHES from a research curiosity to routine buffer shows how demand shapes supply. Companies invest in cleaner, more efficient production methods to lower energy waste and reduce contaminants. Instrument companies and biotech suppliers keep testing purity versus cost, since labs want both. On the science side, researchers focus on tweaking substituents around the cyclohexyl ring to chase different pKa values or make new buffers fit new enzymes or cell types. Funded R&D efforts look for ways to recycle buffer waste, capturing and reusing spent buffer stocks to cut environmental impact. Improvements in chromatography and analysis continue to raise standards for buffer purity, rivaling those seen in pharmaceutical ingredient manufacturing.
Much of the trust in CHES comes from years of toxicity tests with cells and animals. At typical concentrations for lab work, toxicity rates stay low, but higher exposures—like dusts or spills—still pose harm. Cell culture studies note some osmotic effects at high doses, which matters for delicate experiments. Regulatory agencies review toxicity profiles, and that’s filtered down by suppliers into risk labels and storage warnings. With growing concern over lab waste, eco-toxicity has moved into focus. Waste management procedures increasingly require proof that CHES and other sulfonic acids get neutralized or captured before disposal. Real risk stays low for trained handlers, as long as people treat the powder with respect.
Demand for robust buffers won’t fade, especially as researchers dig deeper into protein structure and synthetic biology. New diagnostics and high-throughput techniques constantly test old buffers' limits. Companies already explore greener syntheses, aiming to cut down on toxic byproducts or improve water recycling. Buffers might one day combine with real-time sensor tech, self-adjusting pH on command. As buffer needs shift with next-gen gene editing or cell-based therapies, R&D likely spawns hybrid buffer molecules, tailored not just for pH but for compatibility with engineered enzymes or living tissues. Open collaboration between labs, suppliers, and regulators will help push both safety and science forward, keeping CHES relevant through the next wave of innovation.
2-Cyclohexylamino ethanesulfonic acid, which researchers tend to call CHES, isn't a household name. But ask anyone who's ever stepped into a molecular biology lab, and they’d likely recognize it as a tool found tucked in the chemical cabinet. Most folks, including myself during grad school, encountered CHES while adjusting the pH of a solution for an experiment. In labs, reliable buffers act like referees: they keep everything steady so experiments reflect reality, not chaos.
Let’s talk pH. Many reactions in biology, from protein folding to enzyme activity, rely on a steady pH. Without balance, proteins start misbehaving, data gets messy, and whole research days may go down the drain. That’s where CHES provides real value. This compound's sweet spot is between pH 8.6 and 10, a range not always easy to lock in with other common buffers. Scientists who analyze protein structures, manipulate enzymes, or work on nucleic acids often reach for CHES specifically for this range.
The need for purity and predictability in science pushed the adoption of buffers like CHES. Good Manufacturing Practice (GMP) guidelines and rigorous quality protocols keep research trustworthy. Solid track records count for a lot in science. Reagents such as CHES, referenced in regulatory documents and scientific literature, carry that weight for a reason. Nothing frustrates researchers more than rerunning experiments just because the buffer didn’t deliver what the label promised.
Scientific breakthroughs start small, often with a reliable buffer enabling reproducible data. From gene editing to novel vaccine design, tools like CHES become part of advances that, over time, touch real lives. During the pandemic, for example, diagnostic labs depended on stable, predictable chemistry to track diseases accurately. CHES and its counterparts kept these tests reliable, underpinning decision-making for hospitals and public health agencies.
Not all research labs, especially in developing regions, get consistent access to high-quality reagents. Subpar chemicals cause setbacks, wasted funds, and skewed results. Supporting local and affordable access to certified laboratory chemicals reduces these stumbling blocks. At the same time, handling chemicals like CHES carries inherent risks. Training lab workers, labeling storage, and following waste disposal protocols keeps people safe and the environment protected.
The global research community works best when everyone can trust basic materials. Sharing best practices on sourcing, validating, and safely handling reagents supports better research worldwide. Companies that value transparency and rigorous testing in their supply chains increase confidence and minimize disruptions. Governments and non-profits could do more to support universal access, because fundamental science links back to public health, economic growth, and education.
A single buffer might seem minor, but in science, the small details shape everything that comes after. My own experience using CHES in routine experiments drove home the importance of chemical integrity. Too often, research success pivots on the basics. The story of CHES reminds us of the invisible backbone supporting scientific progress every day.
2-Cyclohexylamino ethanesulfonic acid, known in most labs as CHES, shows up often in biochemistry and molecular biology settings. The first time I handled CHES, the lab manager handed me the bottle with a little speech about “keeping buffers clean,” and it stuck with me. Mishandling a good buffering agent means ruined reactions and wasted hours at the bench.
Reliable sources, including Sigma-Aldrich and contemporary lab safety courses, always outline clear basics for this compound. CHES prefers a dry spot, on the cool side of room temperature. Heat speeds up degradation, and humidity can leave you with clumps in your reagent bottle. Temperatures around 20–25°C seem to give the most stable shelf life. Many labs pick the low shelf of a cabinet, well away from light and moisture.
In damp rooms, CHES tends to pick up water because it’s hygroscopic. One damp bottle, and your accurate mass measurements become guesswork. You pour, thinking you’re measuring active chemical, but quite a bit of that weight might just be water. Reproducibility drops, and your pH calculations can wind up way off track. I once watched a colleague scramble to fix suddenly shifting reaction conditions, only to realize their entire bottle had sucked up some summer humidity. Now, every bottle in our lab has silica packets, and containers stay sealed tight.
Shared storage creates the next pitfall. You start seeing sodium hypochlorite and acids crowding the chemical shelves, and cross-contamination creeps in. Acid vapors, especially, can corrode bottles and react with basic compounds like CHES. Storing CHES with strong acids or oxidizers in the same cabinet nudges disaster closer. In my own work, I picked up the habit from senior staff—designate a separate, labeled shelf for your buffers. The risk of a disaster never feels real until you lose a year’s work over a single tainted buffer.
Labeling sometimes seems basic, but mistakes pile up from unlabeled bottles, which can lead to someone using the wrong chemical by accident. CHES looks like many other white powders, so clear labels with names, concentration, and prepared dates make life easier in a busy lab. I faced an incident where an unlabeled container sent two experiments in opposite directions before anyone caught the mix-up.
Good ventilation helps too. Storing near open windows or in chemical fume hoods keeps air fresh and limits buildup of any accidental dust or fumes. Every lab can set a regular check-up routine, opening each bottle for a once-over: look at the texture, check for color change, see if clumps have formed. If the compound looks off, replace it. The small cost of fresh CHES beats the risk of corrupted results or spoiled experiments.
Posting a checklist covers most issues: keep CHES dry and in a cool location, store away from acids and oxidizers, and use airtight, labeled containers. Simple habits have kept my work clean and reproducible over many years. Strong storage habits also give confidence that every measurement reflects the intended chemical, not mystery water or a contaminant.
Stepping into any research lab, you run into names that sound complicated, and sometimes intimidating. 2-Cyclohexylamino ethanesulfonic acid—often called CHES—shows up a lot in life sciences and chemistry. Usually, it’s used as a buffer chemical, helping to stabilize pH in solutions for experiments and industrial processes. Its popularity has put questions in people’s minds about whether it’s hazardous.
Looking at public safety databases and regulatory resources helps paint a real picture. CHES does not fall in the same category as classic toxic substances. The compound does not emit vapors that burn your lungs. It doesn’t eat through gloves or stain the skin in seconds. Handling the pure, powdered form without proper care—like wearing gloves and goggles—can cause minor irritation to skin, eyes, or the respiratory tract. The Material Safety Data Sheet (MSDS) points out that big doses or long-term exposure aren’t well studied in people, so erring on the side of caution makes sense.
Accidental spills on workbenches or floors won’t cause panic. The dust can cause coughing or minor sneezing, so a dust mask helps. If the powder gets in your eyes, you rinse with water, and most symptoms fade. Swallowing a large quantity—an unlikely event—could irritate your guts and might need medical attention, but doctors don’t categorize this compound as highly toxic.
A lot of common buffer chemicals in labs, like Tris, HEPES, or MES, share similar safety profiles. To put things in perspective, cleaning bleach and ammonia, which many people use at home, are a lot more dangerous. Even table salt in extreme doses can harm, but nobody views salt as a threat in a normal kitchen.
On the scale of lab chemicals, CHES sits in the “handle with respect but not fear” territory. That said, every laboratory should follow basic chemical hygiene—wash hands after use, wear eye protection, keep powders off your face.
Over the years, I’ve prepared CHES buffers for cell biology and protein purification. The experience rings true: it’s not a chemical to be reckless with, but it won’t send you running for the emergency exit. My colleagues and I always wear gloves, avoid eating or drinking near the workstation, and make sure to keep food away from chemical storage.
Disposal procedures reflect its relative safety: you don’t pour gallons down the drain, but small, diluted quantities pass through standard waste streams in many research settings. Still, local rules differ. Most universities and companies tuck it into their “regular non-hazardous waste” bins, just to keep things organized.
Respect for chemicals goes further than checking labels. Training lab workers to recognize symptoms of overexposure, even for less-toxic substances, should stay standard. Facilities should keep Safety Data Sheets within reach, teach colleagues not to mix unknown powders, and keep spill kits handy in case of larger accidents.
Everyone benefits from replacing outdated, hazardous chemicals with safer alternatives. CHES carves a spot among buffers that do their job without drama. Practicing basic safety—proper labeling, secure storage, common-sense hygiene—makes working with chemicals, including CHES, a manageable task for most labs and industries.
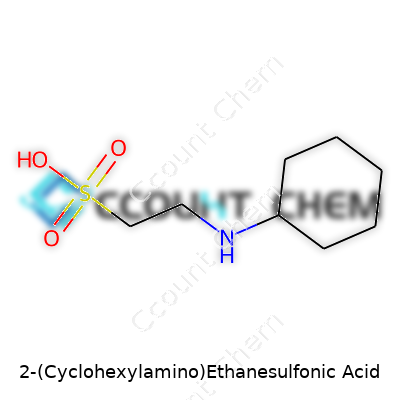
People who work in biology and chemistry labs come across hundreds of buffers, yet few pay much attention to where the numbers and formulas come from. 2-Cyclohexylamino ethanesulfonic acid, better known by the acronym CHES, plays a real role in many experiments that need pH control in the alkaline zone. Talking about its chemical formula, CHES goes by C8H17NO3S. On paper, it looks like just another combination of letters and numbers. Each character and digit points to part of an actual molecule—eight carbons, seventeen hydrogens, one nitrogen, three oxygens, and one sulfur.
Years in the lab have shown me that grabbing a bottle of white powder is the easy part—mixing the right solution depends on knowing your numbers. If you want a reliable buffer, the molecular weight makes all the difference. For CHES, it clocks in at 207.29 grams per mole. Weighing out reagents without knowing the correct value opens the door for pH surprises and unreliable results. Small oversights sometimes mean wasting a day’s work, particularly when an enzyme or protein loses activity because the buffer wasn’t prepared correctly.
CHES shows up in experiments that deal with alkaline pH ranges, usually between 9.0 and 10.0. That’s important in systems such as enzyme assays, protein purification, and even some environmental studies. People sometimes skip over checking if the formula listed on a supplier’s label matches up with what the literature says. More than once, I had to double-check suppliers because two lots from different companies reported varying molecular weights, sometimes due to water of crystallization or impurities. Standard reference sources like Merck Index or Sigma-Aldrich catalogs keep the numbers straight, relying on proper analytical work and peer review. That accountability, along with decades of scientific papers confirming the properties, gives confidence to anyone tuning solutions.
The number printed on the label doesn’t come by magic. Chemical supply chains, regular audits, and batch testing make sure every bottle of CHES contains what it says it does. I’ve run into surprises: a bottle stored in a humid stockroom sometimes contains a little water, pushing the apparent molecular weight higher. Every time you switch a supplier, confirm the certificate of analysis. It cuts down on costly mistakes and helps track down troubleshooting issues later. Checking supplier credentials and reviewing batch documentation supports experimental trust and the kind of reproducibility researchers expect.
Mixing chemical buffers often feels like routine work, but there’s no shortcut for double-checking the numbers. Calculating the right buffer concentration starts with nailing down the precise chemical formula and molecular weight. Use trusted documentation, calibrate scales often, and keep good records. In teams, I found that sharing standardized stock solutions and logging any deviations pays off in reduced error and clearer data. Transparency about chemical batches and weights might seem tedious, but in research and industry, it saves money, time, and reputation. The simple task of reading a label with a critical eye sets the stage for every experiment down the line.
Many people think chemistry is just mixing liquids and waiting for a color change, but choosing the right tools and preparing them correctly often shapes how an experiment turns out. Anyone working with 2-Cyclohexylamino Ethanesulfonic Acid—usually called CHES in a lab—should give this compound the attention it deserves. It’s a buffer, a backbone for a lot of biological science. pH-sensitive experiments run smoother with a buffer like CHES around, and mistakes at the start creep through every phase. From experience, skipping steps at the bench wastes time and materials.
Before measuring anything, check the CHES bottle. Make sure the powder looks dry and white. If you see moisture, the weight will be off. So keep it tightly closed, away from humid air. It feels basic, but contamination from a scoop or dirty weighing paper can lead to cloudy solutions and confusion later. Digital balances with regular calibration block out most weighing errors. No one likes to redo a day’s work because of sloppy prep.
Usually, CHES works as a buffer in the 8.6 to 10 pH range. Finding the correct molarity keeps the experiment on track, so list out every step and concentrate before turning on the tap. When you add powder to water, always use clean, deionized water. A glass beaker helps spot undissolved bits. Pouring straight into volumetric flasks before dissolving causes headaches. The solution clings to the glass and some of the powder won’t dissolve.
Stir the mix well—magnetic stir bars beat manual stirring every time. Cold water takes patience, but it’s better than heating, which can kick off reactions and bring in unwanted breakdown products. My former colleagues would always warn about using hot plates unless you track the temperature tightly. CHES breaks down faster in heat. The only way to make sure of a good result is to keep the temperature steady and the process simple.
After most of the powder dissolves, check the pH. CHES still needs adjusting to hit the sweet spot for buffering capacity. Most use sodium hydroxide or hydrochloric acid and a reliable pH meter, not strips. Rushing pH adjustments throws everything off—the buffer may not hold, and the experiment tanks. If things look off, starting over works better in the long run.
Once you hit the target pH, move the solution to a volumetric flask. Bring it up to volume slowly, mixing as you go, to avoid overshooting. Label the flask with date and concentration. CHES solutions last longer in the fridge and away from light. Marking expiration dates drives good habits, reminds people it’s time for a fresh batch, and keeps everyone safer. I’ve seen labs run into trouble from guessing at how old solutions were.
Careful preparation keeps results consistent. Clean workspaces and thoughtful steps ensure CHES performs as expected. Good records help trace back any problems and show you care about doing honest and careful science. For many in the lab, the preparation isn’t just a chore—it sets the standard and brings trust to every project, large or small.
| Names | |
| Preferred IUPAC name | 2-(Cyclohexylamino)ethane-1-sulfonic acid |
| Other names |
CHES Cyclohexyl-2-aminoethanesulfonic acid N-Cyclohexyl-2-aminoethanesulfonic acid 2-(Cyclohexylamino)ethanesulfonic acid |
| Pronunciation | /tuː saɪkloʊˈhɛksɪlˌæmɪnoʊ iːˈθeɪn.sʌlˌfɒn.ɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 103404-87-1 |
| Beilstein Reference | 1720296 |
| ChEBI | CHEBI:51511 |
| ChEMBL | CHEMBL418476 |
| ChemSpider | 22625 |
| DrugBank | DB07536 |
| ECHA InfoCard | 100.223.552 |
| EC Number | 103404-87-1 |
| Gmelin Reference | 82244 |
| KEGG | C06428 |
| MeSH | D019326 |
| PubChem CID | 89707 |
| RTECS number | GS3710000 |
| UNII | J3T0X6BRZE |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8044265 |
| Properties | |
| Chemical formula | C8H17NO3S |
| Molar mass | 207.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.124 g/cm³ |
| Solubility in water | soluble |
| log P | -2.3 |
| Acidity (pKa) | 9.5 |
| Basicity (pKb) | 7.5 |
| Magnetic susceptibility (χ) | -54.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Dipole moment | 6.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 316.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1022.19 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4164.7 kJ/mol |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 2°C |
| LD50 (median dose) | LD50 (oral, rat) > 5,000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20 mM |
| IDLH (Immediate danger) | Not Established |
| Related compounds | |
| Related compounds |
N-cyclohexyl-2-aminoethanesulfonic acid (CHES) N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) N-cyclohexyl-2-hydroxy-3-aminopropanesulfonic acid (CAPSO) N-cyclohexyl-2-morpholinoethanesulfonic acid (CHMES) |