Some chemicals have a story that winds back through generations. 2-Aminobenzenesulphonic Acid, also known as ortanilic acid, carved out its place in the late 1800s as synthetic dyes took center stage in textile manufacturing. Chemists searching for vibrant, long-lasting colors seized on this compound for its stability and ease of modification. As the decades passed, the demand for consistent color results pushed researchers to refine both synthesis and purification. Industry players moved from small-batch, labor-intensive methods to high-throughput reactors in all corners of Europe and Asia. I remember reading about its early role in azo dye production: factory workers handled sacks of what they called “red oil” powder, often without gloves, as basics like safety data sheets simply didn’t exist. Over time, increased consumer expectations and growing environmental scrutiny led manufacturers to refine technical standards, making the process safer and more sustainable.
You hold a white or lightly yellowish crystalline powder, melting above 300°C, with remarkable solubility in water and a weakly acidic flavor on the tongue (not that you should taste it; those stories from the old dye plants are cautionary for a reason). As a derivative of benzene sulfonic acid with an amine group at the ortho position, this molecule offers two functional handles: sulfonic acid for solubility and reactivity, amine for linking to aromatic or aliphatic groups. Its molecular formula is C6H7NO3S, and it weighs in at about 173 grams per mole. Chemists use specific rotation, high-pressure liquid chromatography, and IR spectroscopy to certify its identity and spot impurities.
On a proper label you’ll find more than just the chemical name: CAS number 88-21-1, hazard symbols sketched in stark black and white, batch and expiry numbers, even country of origin. Some technical users demand purity above 98%, with strict controls on heavy metals, ash content, and moisture. Others want assurance about trace organic contaminants. Workers in plants storerooms avoid open flames and keep the powder in tightly sealed barrels. Compliance with OECD, REACH, and NFPA guidelines brings legal and ethical peace of mind—a lesson hammered home during audits or safety drills. Gloves and breathing protection come off only at the end of a shift.
Old textbooks described pouring fuming sulfuric acid over aniline, carefully controlling temperature and acid ratios to steer the sulfonation to the ortho position. Today’s plants automate much of this, with pumped reactors and feedback sensors limiting runaway reactions. Crude product gets dissolved, filtered, and recrystallized, step by step, to satisfy color and purity demands. Spent acids are neutralized and treated for reuse—a costly requirement, but better than discharging toxic effluent into rivers as in earlier decades. Engineers swap stories about the struggle to squeeze out a few more percentage points of yield, adjusting time, reagent concentrations, and even reactor geometry.
2-Aminobenzenesulphonic acid doesn’t just sit on the shelf; synthetic chemists use it to create dyes, drugs, polymers, and photographic agents. Its amine and sulfonic acid groups participate in coupling reactions with diazonium salts, yielding a rainbow of azo dyes used in everything from wool carpets to plastic discs. Pharmas and agrochemical labs look for reliable intermediates like this because side reactions are well-mapped and straightforward to troubleshoot. Modifications—such as N-alkylation, diazotization, or sulfonation at the meta position—open up new molecules for R&D, each a potential blockbuster or a new failure to learn from.
Walk through a trade catalog and you’ll see this chemical listed as ortanilic acid, 2-aminobenzene-1-sulphonic acid, or sometimes by trade names coined by dye manufacturers. Chemical language can be messy for outsiders, but cross-references and trusted suppliers help avoid confusion—a must for regulatory compliance, especially when shipping across borders.
Eyes sting from even brief exposure to the dust, while chronic inhalation can trigger respiratory distress. Industrial health standards recommend closed transfer systems, local exhaust ventilation, and regular air monitoring. Material safety data sheets outline steps for spills, medical emergencies, and safe waste disposal. The emphasis on proper handling came too late for some older workers, reminding us that a robust safety culture grows from vigilance, not compliance checkboxes alone.
Most production today vanishes into the world of synthetic dyes—textiles, leather, plastics, inkjets. Printing houses once obsessed over shade and fastness; now, attention has shifted to environmental toxicity and allergenic breakdown products. 2-Aminobenzenesulphonic acid also slips into drugs as a building block, sometimes for sulfa antibiotics or diagnostic agents. And in water treatment and electroplating, its solubility and tendency to bind metals turns it into a useful chelating agent, pulling out trace contaminants or acting as a buffer in large-scale baths.
Research teams keep diving into new uses—novel antioxidants, corrosion inhibitors, polymer additives. The structure lends itself to tweaking, so academic papers and patents keep piling up. Scientists study molecular docking, exploring whether the sulfonate and amine groups can disrupt pathological protein interactions or speed up catalytic cycles. Instrumental chemists run LC-MS or NMR scans to check modifications, while toxicologists probe for unintended breakdown products.
Modern studies measure acute and chronic toxicity in laboratory animals, searching for reliable LD50 data and tracking environmental persistence. Wastewater rules have pushed producers to design new synthesis methods with fewer nasty byproducts. Some countries now require predictive modeling for bioaccumulation, and regulatory filings must disclose degradation pathways. Next-gen research hopes to trace every molecule’s environmental fate, moving toward greener dyes and pharmaceutical intermediates with less hazardous legacy.
Refining greener synthetic routes with less waste looks set to drive the next round of innovation. New catalysts and biotechnological approaches offer the chance to shrink pollution and energy costs. Digital modeling helps chemists anticipate potential toxicities before a prototype even gets to the bench. Trends favor bio-based feedstocks and closed-loop processes. From the dye house floor to university labs, the drive continues for safer, purer, more sustainable aromatic sulfonic acids—meeting rising legal standards and a public that cares about how even the smallest chemical choices echo across the globe.
2-Aminobenzenesulphonic acid, sometimes called ortanilic acid, features in the background of plenty of products found at home and at work. I'd probably never heard of it until I started looking into how everyday colors in fabrics and inks actually get made. Behind every vibrant t-shirt or ballpoint pen, chemistry like this shoulders the real workload.
The strongest link with 2-aminobenzenesulphonic acid centers on dyes, especially azo dyes, which make up one of the world’s most common classes of synthetic colorants. Textile companies lean heavily on these dyes to bring lively shades to cotton, wool, and nylon fabrics. Without this acid, the bright blues, reds, and yellows lining store shelves wouldn’t be as consistent or affordable. Manufacturers prefer this compound because it reacts easily, sticking onto dye structures, and that leads to both longer-lasting and bolder colors. That matters for customers who expect jeans to stay blue after several washes.
Papermakers, ink suppliers, and even some plastic producers rely on color chemistry rooted in 2-aminobenzenesulphonic acid. Companies choose it to help pigments hold firm to surfaces, not wash off at a hint of water, and resist fading in sunlight. On the workplace side, I’ve seen its influence reach beyond simple color. Some advanced printing tech, like inkjet and security inks, calls for ultra-stable colors that don’t bleed or smear—here, too, this acid lends chemical stability and performance.
Medications contain surprising recipes, often building complex molecules in several steps. During pharmaceutical manufacturing, chemists use 2-aminobenzenesulphonic acid as a “starter” or intermediate to assemble certain prescription drugs. It helps build molecules for treatments targeting pain, inflammation, and sometimes metabolic issues. While you won’t see this ingredient listed on a medicine bottle, it plays a role in the chain of synthesis that leads to products with real impact on health and safety.
Plenty of people never give a thought to the plastics, textiles, or inks used day to day, but the impact of their production gets larger every year. Compounds like 2-aminobenzenesulphonic acid support industrial coloring and medicine, but they’re not risk-free. Production leaves chemical residues, and improper disposal lets these substances slip into waterways, where they can linger. Surface water tests around certain industrial areas often detect traces left from colorant-making plants. In my local area, environmental groups work with companies to upgrade wastewater treatment, keeping these chemicals out of rivers and drinking water. Regulations in some countries already require factories to capture and treat waste containing sulphonic acids, and technology exists—things like advanced oxidation or membrane filtration—to cut environmental risk. The challenge boils down to enforcement and incentives, especially as growing economies pick up more production work.
Science doesn’t stand still; better options continue to emerge. Researchers look for alternative dye intermediates that break down faster or use less energy to produce. These green chemistries, designed from the ground up to reduce toxicity and waste, promise to handle what 2-aminobenzenesulphonic acid does, but with fewer downsides. Choices in the lab may seem far from daily life, but safer chemistry brings real benefits—all down the supply chain to us. Supporting brands and local policies that focus on responsible chemical use feels like a real way forward. It takes technical skill, investment, and public attention, all working together, to make sure today’s useful chemistry stays an asset and not a threat.
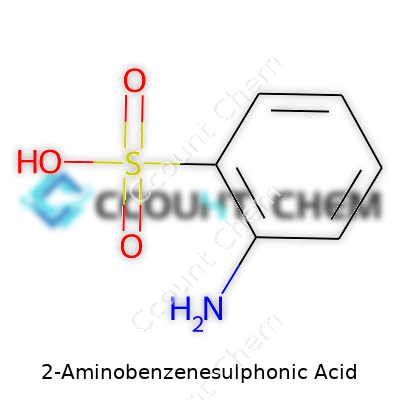
If you’ve tinkered around in a chemistry lab or browsed through a bottle of dyestuffs, you’ve likely crossed paths with 2-aminobenzenesulphonic acid. Its formula looks simple on paper: C6H7NO3S. Behind that formula, though, stands a world of practical use, health conversations, and even questions about sustainability.
A lot of folks look for the catch in chemical names. In 2-aminobenzenesulphonic acid, “amino” means there’s an -NH2 group stuck to the benzene ring. “Sulphonic acid” tells us about the -SO3H group. Attach those groups at the right spots, and you get this formula: C6H7NO3S. The trick lies in arrangement — the amino group attaches at the second position on benzene, giving the compound its unique properties.
It’s easy to dismiss a chemical formula as the stuff of textbooks, but that attitude ignores the pounds and gallons used every day. This compound plays a big role in the dye and pharmaceutical industries. It helps carry color deep into fabric and keeps medicines effective. Its popularity isn’t just from ease of synthesis; it’s because the structure delivers reliability batch after batch. Factories worldwide count on the same reactions to happen every time.
Working with chemicals means thinking beyond utility. The safety sheet for 2-aminobenzenesulphonic acid asks workers to suit up with gloves and goggles. Dust can irritate lungs or skin, and waste run-off can threaten waterways. Handling this compound well keeps the workplace and the planet safer. Regulations from the European Chemicals Agency and the Environmental Protection Agency call for responsible disposal and careful tracking.
Commercial 2-aminobenzenesulphonic acid often starts with benzene, a notorious player linked with health risks if not handled properly. Factories need to trace where their benzene comes from, and chemists face pressure to use less toxic ingredients when they can. Green chemistry alternatives remain a topic for labs and business meetings alike. Sticking with tried-and-true formulas can’t mean ignoring the shift toward safer options.
Transparency matters, especially as consumers and clients start asking harder questions about what goes into their products. Companies can invest in better filtration and waste treatment systems. Researchers try out new catalysts that trim hazardous byproducts. Adopting safer practices isn’t just about ticking boxes; it builds trust and can save money when incidents or spills get avoided.
Education shapes how people think about chemicals like 2-aminobenzenesulphonic acid. Tools like Safety Data Sheets, open-access journals, and even YouTube explainers pull back the curtain for folks outside the chemistry field. This matters for students in classrooms, advocates looking into environmental impacts, and workers just starting to handle chemicals. Real understanding grows when details like C6H7NO3S actually mean something in daily life.
The future of chemicals doesn’t just sit in labs or boardrooms. Public conversation, regulatory pressure, and scientific curiosity push the industry toward safer, more responsible production. Organizations big and small can back education, transparency, and innovation. The formula for 2-aminobenzenesulphonic acid won’t change overnight, but new solutions can shape a better tomorrow for everyone involved.
Most folks haven’t come across 2-Aminobenzenesulphonic acid unless they work in a lab coat or have reason to read chemical labels closely. Known in manufacturing as ortanilic acid, this white crystalline powder turns up in dye and pigment production, and sometimes in specialty paper, rubber, and pharmaceuticals. The minute you hear “used in dyes,” concerns crop up about what happens if a spill occurs or the powder escapes the factory floor.
I once worked in an industrial warehouse where chemicals, including acids like this, lined dusty shelves. Direct skin contact with 2-Aminobenzenesulphonic acid may cause mild irritation—itching or redness, similar to what you might get if you brush against fiberglass. It’s not as harsh as hydrochloric acid, but it isn’t as benign as plain salt, either. Eyes fare worse; splashes can sting and cause discomfort, so goggles are crucial if you’re handling it.
Dust or fine particles in the air can irritate the nose or lungs. Workers exposed to powders like these sometimes describe a bitter, metallic taste or a scratchy throat after hours in a poorly ventilated workshop. It’s not a substance you want floating in the air, especially if you have asthma.
Swallowing 2-Aminobenzenesulphonic acid is rare but possible through accidental contamination. Symptoms like nausea and upset stomach tend to show up, and medical attention becomes necessary if someone swallows more than a pinch.
If a bag breaks in a warehouse, the main worry is keeping the dust out of the lungs and off the skin. Quick cleanup with gloves, a mask, and proper disposal is standard. On the factory floor, where I’ve seen spill kits on standby, the process is swift—scoop, bag, seal, and label for disposal. No one wants to track that powder from shop floor to break room.
Outdoors, water runoff is a concern. Like many industrial chemicals, improper disposal can upset local waterways and harm fish or aquatic bugs. Environmental regulations typically push facilities to install containment. Growing up near a chemical plant, I remember hearing about traces of chemicals in streams and the community worry that followed.
Let’s turn to what the pros say. The Globally Harmonized System (GHS) on chemical hazards doesn’t put 2-Aminobenzenesulphonic acid on the same danger level as strong acids or heavy metals. Most safety datasheets list it as an irritant but stop short of calling it a toxic threat at low levels. Long-term, there’s little evidence pointing to cancer or serious health effects unless there’s regular, heavy exposure—something unlikely outside industrial settings.
This information matches my own experience, and also the guidelines put out by agencies like OSHA. Respect for the chemical, good ventilation, gloves, goggles, and quick cleanup take care of most safety issues.
Chemical safety at work starts with training. Signs reminding workers to wear protection, regular safety drills, and easy access to washing stations matter more than fancy warning labels. Using sealed equipment and vacuum systems rather than open handling reduces dust. I’ve seen success using powder containment boxes where workers transfer small amounts safely. Waste should end up in a labeled, sealed bin rather than the regular trash.
For communities near plants, transparency builds trust. Clear information about what’s produced, how it’s handled, and what happens if a spill occurs helps residents feel safer. Local water monitoring and emergency plans show that safety isn’t just a paperwork exercise—it’s an everyday commitment.
2-Aminobenzenesulphonic acid stands out as a staple in dye manufacture and chemical synthesis. Keeping it in a workspace means taking real steps to keep risks low, since improper storage exposes workers to unnecessary danger and can ruin valuable stock. My own experience in a university lab made one fact clear: chemical safety grows from clear storage routines, not from chance.
The main risk comes from 2-Aminobenzenesulphonic acid’s tendency to irritate skin, eyes, and airways. Inhalation of dust can cause respiratory issues. Spills damage surfaces and force time-consuming cleanup jobs. I once witnessed a small bottle left open overnight; the contents clumped up from moisture and the staff had to run a fume hood for hours to clear the smell. Preventing mistakes starts with the right setup.
Cool, dry, well-ventilated cabinets protect this chemical best. Keep it away from direct sunlight, which encourages degradation and generates byproducts that pile up as dust or sticky residue. I always set chemicals like this on a dedicated shelf, away from acids and oxidizing agents. Cross-contamination sparked one of the worst near-misses I’ve seen, so separation matters.
Humidity is the real enemy. Moisture seeps in and ruins this acid, making it clump or cake. To fight that, airtight containers with tight-fitting lids work well. Silica gel packets add a barrier against moisture, protecting the chemical even during humid spells. In my workspace, a bag of silica gel lasts for weeks, soaking up humidity before it can do any harm.
Over the years, I’ve lost track of how many times poor labeling led to confusion. Clear, bold labels in the local language leave no doubt about what sits on a shelf. List both the full name and any common nicknames. Date new stock on arrival. Rotate inventory so older batches get used up first.
Chemicals, especially those with recognized hazards, shouldn’t be left where anyone can grab them. Lockable cabinets that open only for trained staff cut down on careless handling. In my lab, a log book tracks sign-out and return of sensitive chemicals, building accountability and reducing mistakes.
Even the tightest setup can’t always stop an accident. Keeping spill kits right next to chemical stores saves precious time. An absorbent powder, a scoop, safety gloves, and a waste bin make cleanups fast. Trained staff react calmly because they know that scrambling for supplies eats up time during a spill.
Emergency showers and eyewash stations belong in any room where hazardous chemicals get stored. Proper storage reduces the need for these tools—but knowing where to find them means workers don’t get caught off guard.
Storing 2-Aminobenzenesulphonic acid safely shouldn’t feel tricky or overwhelming. Focus on dryness, good labeling, and restricted access. Spill kits and clear procedures back these efforts up. Drawing on real-world experience and established chemical safety guidelines, it’s clear that consistent habits make storage safer, protect health, and cut down on wasted inventory.
A lot of labs and industries rely on compounds like 2-aminobenzenesulphonic acid. This chemical, also known as orthanilic acid, forms the backbone of many dyes and chemical processes. Its structure brings out certain physical properties worth noticing, especially for folks with hands-on experience in chemistry labs or product engineering.
2-Aminobenzenesulphonic acid shows up as a white or off-white powder. It’s not flashy, but it’s practical. The powdered form makes it easy to measure and blend. Unlike some greasy or sticky chemicals, this one doesn’t clump, and it spreads evenly in mixtures, which saves time during weighing and mixing. The granules don’t absorb moisture quickly from the air, so it flows freely from bottle to beaker—no chiseling or shaking required.
In water, 2-aminobenzenesulphonic acid dissolves easily, forming clear solutions. This solubility opens doors for dye production and research use. It’s not soluble in organic solvents like ethanol or ether, so water-based processing always gets the job done. Whenever I’ve mixed it, I noticed the acid doesn’t froth or release any odd scents on contact with water, which makes life easier for anyone working on large-scale reactions.
The melting point sits high, around 300°C, and it decomposes before melting. This high thermal threshold proves useful in industrial dye baths, where elevated temperatures and strong chemicals come into play. No worrying about the compound breaking down during normal heat cycles. That thermal stability delivers a peace of mind to technicians running day-long operations.
The compound lands on the denser side, with a relative density of about 1.485 g/cm³. The density helps with predictable storage and shipment, letting logistics teams get accurate weight estimates and avoid surprises. If stored in a dry container away from direct sunlight, this acid stays chemically unchanged over long periods. No yellowing, no caking—just a steady supply ready for future use. I’ve seen jars stored for years without issue, as long as basic dryness and sealing standards were followed.
Many industries rely on dyes for textiles, paper, and plastics. The physical traits of 2-aminobenzenesulphonic acid keep production running efficiently. Because it dissolves smoothly in water, workers don’t deal with clumps or partial dissolutions that would slow down mass production. Safe handling means less training time and fewer workplace accidents. The absence of persistent dust or odor helps protect workers’ lungs and noses. In my time overseeing large lab batches, I watched how new hires quickly adjusted to handling this powder without fuss or complicated gear.
On the environmental side, predictable storage life reduces chemical waste. Companies avoid throwing out old product, which helps save money and keeps disposal manageable. Well-packed shipments face fewer issues with spillage or degradation, which also matters for safety along the supply chain.
Some challenges remain. Handling large volumes safely and ensuring containers stay dry demands attention from everyone involved. Investing in airtight packaging and training remains essential. Exploring greener disposal options can further improve safety and sustainability. By respecting the physical properties of 2-aminobenzenesulphonic acid, both industry and the environment can benefit from efficient, straightforward chemical processes.
| Names | |
| Preferred IUPAC name | 2-Aminobenzene-1-sulfonic acid |
| Other names |
Sulfanilic acid 2-Aminobenzenesulfonic acid Benzenesulfonic acid, 2-amino- o-Aminobenzenesulfonic acid 4-Aminobenzenesulfonic acid |
| Pronunciation | /tuː əˈmiːnəʊˌbɛnˈziːnˌsʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-21-1 |
| Beilstein Reference | 1209229 |
| ChEBI | CHEBI:17234 |
| ChEMBL | CHEMBL285325 |
| ChemSpider | 12227 |
| DrugBank | DB03351 |
| ECHA InfoCard | 03c2c608-2a4e-409f-81ba-bb08aab80a40 |
| EC Number | 3.1.3.7 |
| Gmelin Reference | 82644 |
| KEGG | C02325 |
| MeSH | D08.811.277.040.340.024.200 |
| PubChem CID | 978 |
| RTECS number | DA6475000 |
| UNII | 7O8F81W9N5 |
| UN number | 2585 |
| Properties | |
| Chemical formula | C6H7NO3S |
| Molar mass | 173.19 g/mol |
| Appearance | White to light brown crystals or powder |
| Odor | Odorless |
| Density | 1.485 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Vapor pressure | 0.0000133 hPa (25 °C) |
| Acidity (pKa) | -3.0 |
| Basicity (pKb) | 3.68 |
| Magnetic susceptibility (χ) | -72.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.654 |
| Viscosity | 700 cP (20°C) |
| Dipole moment | 3.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 175.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -472.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1486 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A16AX13 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P260, P264, P270, P271, P301+P312, P304+P340, P312, P330, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 138 °C |
| Autoignition temperature | 485 °C |
| Lethal dose or concentration | LD50 oral rat 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 5000 mg/kg |
| NIOSH | B123 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
Aniline Benzenesulfonic acid Sulfanilic acid p-Aminobenzenesulfonic acid (para-Aminobenzenesulfonic acid) o-Nitrobenzenesulfonic acid m-Aminobenzenesulfonic acid |