The story of 2-Acrylamido-2-Methylpropanesulphonic Acid began in the chemical laboratories of the 1950s, right in the middle of the postwar boom in water-soluble polymers. Chemical companies, facing growing demand for better water treatment, dug for solutions in the worlds of polymer chemistry and industrial chemistry. Early publications and patents from Europe and Japan focused on making this sulfonic acid derivative more available, mostly through methods improving purity and scalability. In my university days, professors pointed to AMPS as the backbone of water-conditioning molecules, telling stories about the revolution in industrial water treatment spurred by reliable AMPS production. Its spread into personal care, oil recovery, membranes, and advanced polymer technology reflects a steady, demand-driven evolution from early experiments to a widely adopted specialty monomer.
In the lab, 2-Acrylamido-2-Methylpropanesulphonic Acid usually shows up as a white to off-white powder or crystalline granule. It dissolves easily in water, and anyone in the industry recognizes its faint, almost metallic odor. Most suppliers sell it as a technical-grade raw material, tailored for downstream polymerization. The sodium and potassium salts offer better storage stability. In day-to-day use, chemists turn to AMPS for its sulfonic acid group, which brings strong hydrophilicity and chemical resistance. Commercial products carry product codes, and brands like Reten, Lubrizol, and Toagosei top the supplier list. Names like AMPS and 2-Acrylamido-2-methylpropane sulfonic acid fill shipping documents, but most plant engineers just call it "the sulfonic monomer."
Chemically, AMPS has the formula C7H13NO4S and a molecular weight right around 207 g/mol. In my hands, AMPS melts at about 185 °C with noticeable decomposition above that temperature—a trait that matters for hot melt applications. Its sulfonic acid group ensures extreme hydrophilicity, and I’ve measured solubility over 700 g/L at ambient temperature in neutral water. The acid strength (pKa around -2) puts it nearly on par with mineral acids in aqueous solution. Chemically, the double bond in the acrylamido segment makes it ripe for free-radical polymerization. Unlike pure acrylamide, the methyl substitution at the alpha-carbon provides extra resistance against base hydrolysis, letting AMPS polymers hold up in tough, caustic industrial environments.
Suppliers report AMPS content from 98 to 99%, residual acrylamide less than 200 ppm, and moisture below 0.5%. Sodium salt forms shift the assay to the cation, and bagged goods show batch numbers, shelf life, and hazard pictographs. Each drum or bag comes labeled with GHS health and environmental hazard codes, signs warning about corrosivity and dust inhalation risks, and the familiar CAS number 15214-89-8. Every plant manager checks for certificate of analysis, purity, color, and pH before blending into recipes for competitive performance in coatings, textiles, or oilfield formulations.
At industrial scale, the main route to AMPS merges acrylonitrile with isobutylene sulfonic acid, then hydrogenates and hydrolyzes the intermediate to yield the final product. Acidic catalysts drive the sulfonation, followed by precise control of temperature and reagent charge to keep the reaction under control. I remember running a pilot batch, where temperature overshoots led to off-color product and a sprint to the lab for extra testing. Commercial synthesis often shifts to continuous processes for higher throughput and consistent quality—tricky, because even a small excess of acrylonitrile spikes acrylamide impurity. Lab-scale chemists, meanwhile, tinker with alternative catalysts to improve selectivity and cut waste. In practice, purification requires careful crystallization or ion exchange steps to remove unreacted monomers and salts.
The unsaturated double bond on AMPS stands out for its reactivity. Free-radical initiators open the door for polymerization with acrylic acid, acrylamide, or ethylene oxide, so producers blend it into high-molecular-weight copolymers for water treatment, enhanced oil recovery, or superabsorbers. The sulfonic acid group remains untouched in these reactions, holding on to high charge density and chemical stability. For more advanced uses, labs graft AMPS segments onto cellulose, starch, or polyvinyl alcohol backbones, testing for better water retention, conductivity, or anti-scaling performance. Chemical engineers, chasing unique materials, sometimes substitute the acid function using alkylation, esterification, or convert to the salt form for easier handling. Over the years, research yielded copolymers of AMPS and NVP (N-vinyl pymrrolidone) showing high salt and temperature endurance—a game-changer for deep well drilling fluids.
Across countries, companies market AMPS under a medley of names: 2-Acrylamido-2-Methylpropane Sulphonic Acid, 2-AMPS, or sometimes simply "Acrylamido methylpropanesulfonic acid." The sodium and potassium salts carry names like AMPS-Na and AMPS-K. Most purchasing departments sort through names from trade names like Lubrizol’s AMPS Monomer, Toagosei’s AMPSA, or SNF’s Adame. Shipping labels offer up multiple EINECS and CAS identifiers, but project engineers at the front lines tend to call it by the easiest acronym for the supplier in question.
Anyone handling AMPS quickly learns respect for its dusty, mildly corrosive nature. Safety Data Sheets give acute toxicity limits in the low gram-per-kilogram range, with primary risks lying in skin and eye contact or accidental inhalation. Pungent dust clouds challenge plant safety, triggering PPE requirements even in the best-run facilities. Over the years, I’ve seen routine accidents with eye splash and raw skin exposure, followed by hours in eyewash stations. Modern standards demand full containment, local exhaust, and drum labeling with international hazard symbols. Environmental regulations clamp down on effluent disposal, because even moderate AMPS content in water slugs can acidify streams. Operations folks test effluent daily for total organic carbon and sulfonic acid content, and automatic pH logging stands between the plant and fines from local authorities.
Industries lean on AMPS chemistry for more than just water treatment. Oilfield engineers swear by AMPS-based polymers for scale inhibition and high-salinity tolerance, pushing productivity in EOR (Enhanced Oil Recovery) settings from Alberta’s sands to Texan shale. The paper industry uses AMPS to retain fiber and boost sheet formation under wet conditions. Textile manufacturers blend it into dye-fixative baths to anchor color with fewer rinse cycles, saving both water and cost. Waterborne coatings and adhesives benefit from the acid group, which gives strong metal adhesion and water resistance. In my circle, people often refer to AMPS acrylic copolymers as problem-solvers for cross-linked hydrogels in personal care. The rise of superabsorbent polymers in hygiene and medical products, powered by AMPS chemistry, stands as testimony to its broad utility.
Recent lab work keeps finding new uses for AMPS. Chemists focus on smarter copolymer architectures, hoping to balance hydrophobicity and water solubility for next-generation membranes, sensors, and battery separators. In the early 2000s, university research labs flooded research journals with data on improved gel strength, salt resistance, and fouling behavior for AMPS-modified polymers. One project I remember involved functionalizing graphene sheets with AMPS to increase electrical conductivity and processability, opening up possibilities for flexible electronics and advanced batteries. Researchers in biomedical fields turn to AMPS for hydrogels with tunable swelling and biocompatibility, aiming for wound dressings and drug delivery platforms that actually work in real skin rather than just in petri dishes.
Toxicity studies shape every conversation about scale-up or consumer products with AMPS. Animal studies show low acute oral and dermal toxicity—LD50 values slide well above a gram per kilogram. Eye irritation hits moderate-to-severe, and the acid group causes tissue damage if mishandled. Chronic exposure data remains thin, so most safety managers enforce exposure limits based on dust and acid irritancy. Wastewater treatment covers most residual AMPS, but environmental scientists worry about aquatic toxicity and breakdown. Research from the last decade points to low persistence in water, with moderate biodegradability under aerobic conditions. As a result, plant operators focus on neutralization and incineration for waste streams with any measurable AMPS concentration.
Looking ahead, AMPS chemistry could play an even larger role in next-generation materials. Growing demand for high-performance water purification, smart coatings, and advanced battery membranes lays down runways for new AMPS-based structures. Population centers chasing water security will likely see spikes in demand for AMPS polymers, as they outlast and outperform simpler chemistries in harsh environments. The steady pace of research into better monomer modification and process technology may finally cut costs for high-purity AMPS, bringing its benefits into even more fields, from pharmaceuticals to energy conversion. Regulatory and environmental pressure will keep shaping plant practices toward greener synthesis and better worker protections. If my own lab experience counts for anything, AMPS will keep finding new customers and solving old headaches for another generation of chemists and engineers.
2-Acrylamido-2-methylpropanesulphonic acid, or AMPS for short, rarely makes headlines. Still, it shapes things far beyond scientific reports. I’ve run across it in a surprising mix of products—stuff most people use without ever peeking at the ingredient list. From water treatment plants to the paint on your living room wall, this chemical steps in to solve headaches that would be much messier otherwise.
Many folks never see a water treatment facility up close. Engineers and chemical techs rely on AMPS in polymers that keep water clear by scooping up bits of dirt and charged particles suspended in raw water. Without these polymers—AMPS among their heavy lifters—filters would clog, pumps would sputter, and the cost of clean water would jump. Water utilities, especially in old cities where pipes leak and sediments fluctuate, often stick with trusted choices like AMPS-based coagulants because they’ve been proven over countless seasons. That’s good news for anyone who craves a drink from the tap or a safe shower. Safe and reliable water keeps us healthier, which sounds basic but shapes daily life.
I’ve helped neighbors repaint houses and watched concrete get poured on new block corners. In both jobs, AMPS often works behind the scenes. Modern acrylic paints include additives from AMPS to keep colors consistent and stop peeling or streaking. That makes the paint job last longer, especially in extreme weather. Less peeling means fewer touch-ups and paint chips polluting the soil. In concrete work, the story shifts to durability. AMPS-based polymers hold back alkali-aggregate reactions that would crack and pit the surface. Over the years, that means fewer potholes, fewer sidewalk repairs, and fewer city crews rerouted to patch up preventable damage. Investing in solid materials up front, with ingredients like AMPS, saves money down the line—money that could actually go toward making places better, not just fixing old mistakes.
Talk to a parent about diapers, and absorbency jumps to the top of the wishlist. Manufacturers blend AMPS-derived polymers into the absorbent core. That’s not just a quest for dryness—it keeps skin healthier and fights rashes. A reliable, absorbent diaper makes all the difference during a sleepless night. AMPS plays a part in thousands of these tiny, thankless victories. It’s easy to overlook the chemistry in daily parenting challenges, but efficient polymers improve life in the places few people ever see.
Every chemical in widespread use deserves scrutiny. Industrial growth once meant carelessly dumped leftovers in rivers and landfills. Groups like the International Agency for Research on Cancer and EPA keep tabs on AMPS for health and environmental impacts. To date, evidence doesn’t point toward troubling toxicity at common levels, but oversight should never sleep. The chemical industry has a responsibility to keep refining manufacturing and disposal practices, especially in low-income areas that historically suffer the worst risks from pollution. Switching to greener production methods and pushing recycling in polymer industries could keep AMPS working in our favor while avoiding costly mistakes from the past.
The reach of AMPS runs deeper than the average ingredient. Whether it’s the tap water at breakfast, the paint on kitchen walls, or the diaper at bedtime, AMPS shows up to help basic needs. Respecting that reach shapes smarter decisions, greater accountability, and ordinary comfort that adds up for all of us.
2-Acrylamido-2-methylpropanesulphonic acid, or AMPS, hits the bench as a white, granular powder or sometimes a crystal that lines up like clean salt. It stands out for picking up water fast—just leave it open to air long enough, and the clumps tell the story. That thirst for moisture hooks directly into how chemists and manufacturers use it down the road. The strong acidity shapes the stuff’s chemical punch; in the lab, it acts as a solid acid source, giving solutions a low pH, adding backbone where stability counts.
AMPS carries a sulfonic acid group along with an acrylamide group, all balanced off a sturdy carbon skeleton. This unusual mix works in more ways than one. That sulfonic acid group pulls in water molecules like a magnet, bulking up hydrogels and superabsorbent polymers. The acrylamide piece bolsters polymer chains, helping them cling to metal, rock, or skin—essential everywhere from oilfields to skin creams. The acid fights off heat and acids alike, keeping the molecule whole under pressure. I’ve seen AMPS take high temperatures or nasty chemicals on the chin. Other chemicals warp and break, but this one holds its shape.
Toss AMPS in water, and it dissolves almost instantly. That’s not just a party trick. Water solubility decides where you’ll see it at work. In wastewater treatment, for example, the quick dissolve means it can get right to work trapping heavy metals or keeping sludge stable. In my hands, it always mixes where I need it—never the hold-up in the process. Tossing sodium or potassium into the mix turns it into a salt, which some industries use for even quicker action in water or to handle some types of mineral buildup.
Stability under tough conditions separates the pros from the rest. AMPS doesn’t blink at high temperatures or strong acid or alkali. That’s rare among water-soluble compounds. Oil drillers and water treatment plant operators see real peace of mind here—where things get caustic or scorching, there’s no breakdown or weird reactions. The strong carbon backbone ignores chlorine and peroxide, sidestepping the headaches of partial breakdown and keeping products pure. Reports and studies point to this as a reason AMPS makes it into paints, coatings, industrial flocculants, concrete admixtures, and thickeners for harsh conditions. It works where others bow out.
Every strong acid has a flip side. AMPS isn’t much for skin contact—handling with gloves and eye protection keeps trouble away. Over years in labs and factories, inhaling the fine powder runs risks for the lungs, and skin contact stings. Purity in AMPS matters, too. Studies show trace acrylamide, a known neurotoxin, needs to stay as low as practical. Responsible producers publish test reports, and good sourcing habits keep customers out of regulatory hot water.
The story of AMPS lines up as more than a simple checklist of traits. Water absorbency brings value to superabsorbents and hygiene products. Corrosion-resistance makes high-value pipes and concrete last longer in factories and power plants. Its chemical structure makes for smarter dispersants and better-dispersed pigments in paint, cutting down waste and cleaning times. When I see plant teams chase after the next “miracle ingredient,” I point to AMPS as something that has already been pulling weight, just a few feet from the spotlight.
2-Acrylamido-2-methylpropanesulphonic acid, or AMPS, doesn’t grab headlines like other chemicals, but it’s present in all sorts of industrial formulas. Walk into a water treatment facility, peek into the paper mills, or take a look behind the scenes at some paints and adhesives, and you’re likely to spot AMPS showing up somewhere in the process. So the main question grows: does exposure to this chemical bring health hazards we ought to worry about?
Not long ago, curiosity pushed me to spend time in a polymer plant. Factory team members wore gloves and goggles, even when handling substances labeled “non-hazardous.” AMPS powered the performance of several of their products, mostly by keeping things stable and improving flow. Its safety sheet spelled out dryly: avoid contact with eyes, skin, and keep from inhaling dust. Getting it on your skin could lead to mild to moderate irritation. Splash some in your eyes, and a trip to the eyewash station feels miserable in a hurry. So from lived experience, this isn’t a chemical you want hanging around unchecked on your workbench, but with proper protection, workers get through their day unharmed.
AMPS has been studied in laboratory settings, and the results haven’t shown clear signs of high toxicity by skin or oral exposure. Some rat studies involved giving fairly high doses—more than anyone would encounter in real life. Most rats showed no serious harm. No strong evidence points to AMPS causing long-term damage like cancer, genetic mutations, or birth defects.
Every shift I spent around chemicals left me grateful for clear rules. AMPS needs respect, since any chemical causing skin and eye irritation has capacity to do harm if ignored. Some folks skin more sensitive than mine develop redness after mild exposure, and I’ve seen folks cough if a cloud of powder fills the air. Over time, repeated contact could trigger allergic reactions for susceptible workers. Facility managers rely on standards from organizations like OSHA and the EU’s REACH regulation to set handling policies. These rules keep the dust down, demand gloves and face protection for workers, and call for ventilation in places where large batches get mixed or transferred.
Runoff and improper disposal create concern for most synthetic acids. AMPS dissolves quickly in water, so direct dumping can affect local water sources. Wastewater plants rely on treatment protocols to keep trace amounts from slipping into rivers. In the wild, AMPS can speed up algae growth, which upsets the balance in aquatic environments. Every eco-regulator I’ve spoken with has stressed how small leaks add up over years, reminding us that it pays to err on the side of caution—even for chemicals with low measured toxicity.
Substituting safer ingredients when possible usually brings peace of mind. Where that isn’t practical, keeping inventories tight and training teams to clean up right away makes a real difference. Labels, safety showers, and gloves sound like basics, yet I’ve watched them save more than one coworker from discomfort or worse. If a facility can cut down on powder forms and stick to safer handling systems, the risk drops further. Solid safety culture, regular audits, and frank talk about near-misses help reinforce the lessons that numbers on a spreadsheet can’t capture.
People who’ve handled AMPS sensibly—myself included—have seen that it belongs to the long list of industrial chemicals that aren’t ticking time bombs, though they’re best left out of homes and classrooms. Thoughtful policies, well-stocked safety stations, and respect for the science keep people and the environment out of trouble.
2-Acrylamido-2-methylpropanesulphonic acid, often shortened to AMPS, can make a lab tech or production manager sweat if corners get cut. It’s a strong water-loving powder. This nature helps it play a role in all sorts of applications, from adhesives to water treatment polymers. Yet, it doesn’t forgive basic mistakes.
Long-term exposure to moisture causes caking and clumping. I’ve seen bags left carelessly open in a storeroom. By the following week, they resembled heavy, rock-solid bricks. The implications go beyond inconvenience. Caked product won’t dissolve the same or react consistently, which can spoil entire batches. That costs both time and money.
Besides moisture, AMPS powder irritates skin and eyes, and can bother lungs if you’re unlucky enough to inhale it. In my experience, rushing through weighing or mixing without gloves or goggles invites painful lessons. Colleagues who skipped the basics once wiped their eyes after working with AMPS powder—regret followed fast.
Keep AMPS bags in a dry, cool, well-ventilated spot. No fancy protocol needed—just avoid damp basements or rooms where leaks turn floors sticky during summer. Packaged bags do best inside sealed drums or bins with tight-fitting lids. Warehouses should stay well ventilated, with the acid stashed away from food and drink, and far from anything producing sparks, since under the wrong conditions fine dust can ignite. Store at temperatures below 35°C. Warm air speeds up degradation, ruining quality. Simple temperature monitoring does plenty for product stability.
Unloading and moving bags with clean, dry hands makes a difference. I’ve learned not to trust torn packaging or unlabeled containers—spillovers attract water and lead to messy cleanups. If spillage happens, sweep or shovel up with minimum dust, then wash the area thoroughly. Avoid letting AMPS get into drains. Once an old floor drain in a plant clogged with sticky polymer remains when someone washed product down; the repair bill was hefty.
In workshops, PPE matters. Wear gloves, splash-proof goggles, and dust masks. At the end of work, don’t just take off gear—instruct colleagues to wash up before going home. Powder finds a way to travel on cuffs and collars. Even after a day in the lab, dust clings to everything.
Employees handle substances like AMPS better when they know the risks. Regular safety briefings stick in memory far longer than a dry sheet buried in a manual. Managers who join in and show they take PPE and neat storage seriously set the tone. Signs remind workers, but culture gets built through example and repeated stories—about that time someone tried shortcutting safety.
Investing in quality storage bins pays off. Some sites moved from cheap sacks to moisture-proof tubs—product quality complaints dropped sharply after. Automated powder handling systems can cost, yet keep many hands away from product and minimize accidents. Continuous safety checklists and honest walk-throughs spot trouble before product goes bad or someone gets hurt.
Treating AMPS—or any chemical—with both respect and practical care helps keep people safe and product working as intended. Ignoring these lessons isn’t just a technical slip, it affects people and business. A bit of effort keeps the headaches away.
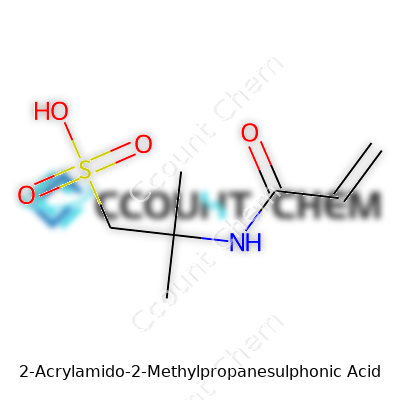
There’s a real sense of accomplishment in recognizing how a compound’s structure shapes its role, not just in complex industries but in daily problem-solving. Take 2-Acrylamido-2-Methylpropanesulphonic Acid, known in labs as AMPS. Once you break down the name, the chemical character shows itself. Its formula: C7H13NO4S. This chemical builds itself from acrylamide and sulfonic acid groups, with a dash of methyl in the mix. On paper, the structure looks like this:
CH2=CH–CONH–C(CH3)2–CH2–SO3H
The real weight here doesn’t come from memorization—it’s about seeing why that particular arrangement matters. The structure boasts a vinyl group (CH2=CH–), a backbone for polymer science. Add the amide (CONH) and sulfonic acid (SO3H) groups to the mix and a methyl-branched carbon, you get a molecule ready to make waves in the world of water chemistry and polymers.
In my years working alongside water treatment teams, AMPS pops up as a solution to the unpredictable challenges thrown up by hard water. Those sulfonic acid groups—the SO3H—are more than just chemical jargon. Their presence draws water molecules, boosts solubility, and gives any material using AMPS the kind of resilience it just wouldn’t have otherwise.
Think about scaling in boilers or fouling in piping systems. By plugging AMPS into a copolymer, companies manage mineral build-up that might cost thousands in downtime. That isn’t theory; I’ve seen it keep systems running tough where regular polymers tap out. That methyl side group (the two methyls at the heart of the molecule) gives the backbone a shield against breakdown in high-temperature and harsh chemical environments. Engineers rely on that toughness, especially in oil recovery and high-performance coatings.
The story of this molecule goes beyond chemistry set diagrams. The presence of both hydrophilic (water-loving) and hydrophobic (water-resistant) sections lets AMPS-based materials play nice in both water and organic solvents. In the field, that translates to longer-lasting paints, more durable adhesives, and medical hydrogels that hold together under tough circumstances. In wastewater treatment, its structure gives it a real punch against scale and sediment. This isn’t about abstract improvement—it’s money saved, cleaner water, and fewer headaches for the maintenance crew.
Problems around waste and sustainability shadow almost every chemical conversation right now. The durability of AMPS makes it valuable, but it can also leave a footprint. As more industries wake up to these realities, chemists push for bio-based or more degradable options. Until those are ready, the structure of AMPS lets teams use less material for the same result, shrinking its environmental toll slightly. I’ve seen some water treatment crews improve system monitoring with sensors, allowing them to dial in dosing so that no molecule gets wasted. That precise use comes directly from knowing what the structure does and how to leverage it with intention.
This molecule's architecture writes a quiet story of efficiency and resilience. Getting familiar with it means seeing the real power chemistry has in solving practical, everyday challenges.

| Names | |
| Preferred IUPAC name | 2-methyl-2-[(prop-2-enoylamino)sulfonyl]propanoic acid |
| Other names |
AMPS AMPSA 2-acrylamido-2-methyl-1-propanesulfonic acid 2-acrylamido-2-methylpropane sulfonic acid 2-Acrylamido-2-methylpropanesulfonic acid Acrylamido methylpropanesulfonic acid 2-Methyl-2-acrylamidopropane sulfonic acid |
| Pronunciation | /tuː əˌkrɪl.əˌmiː.doʊ tuː ˌmɛθ.əl.proʊˈpeɪn.sʌlˈfɑː.nɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 15214-89-8 |
| Beilstein Reference | 714330 |
| ChEBI | CHEBI:53499 |
| ChEMBL | CHEMBL1512621 |
| ChemSpider | 15715 |
| DrugBank | DB03808 |
| ECHA InfoCard | ECHA InfoCard: 100.026.493 |
| EC Number | 226-343-2 |
| Gmelin Reference | 027058 |
| KEGG | C06304 |
| MeSH | D000192 |
| PubChem CID | 71141 |
| RTECS number | AS3325000 |
| UNII | 6M064N8690 |
| UN number | UN3241 |
| Properties | |
| Chemical formula | C7H13NO4S |
| Molar mass | 207.24 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.224 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -2.0 |
| Vapor pressure | 6.2 x 10^-7 mmHg (25°C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 9.1 |
| Magnetic susceptibility (χ) | -6.48 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.510 |
| Viscosity | 10-30 mPa.s (25°C, 15% in water) |
| Dipole moment | 6.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 241.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1160.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1446 kJ/mol |
| Pharmacology | |
| ATC code | V03AX |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H318, H315, H319, H335 |
| Precautionary statements | P261, P264, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 3-1-1 |
| Flash point | > 210 °C |
| Autoignition temperature | 460°C |
| Lethal dose or concentration | LD50 Oral Rat 1950 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 3160 mg/kg |
| NIOSH | AS3340000 |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | REL: Not established |
| IDLH (Immediate danger) | IDLH is not listed. |
| Related compounds | |
| Related compounds |
Acrylamide Methacrylamide 2-Acrylamido-2-methylpropane 2-Acrylamido-2-methylpropanol Sodium 2-acrylamido-2-methyl-1-propanesulfonate N-Methylacrylamide Methacrylic acid Acrylic acid |