Digging into the roots of 2-Acrylamido-2-methylpropane sulfonic acid (AMPS), the story rolls back to mid-twentieth century chemistry. Early polymer scientists wanted water-soluble monomers that brought more to the table than the usual suspects. They searched for a combination of robust ionic groups and solid backbone stability. AMPS stepped up to the challenge, thanks to deep experimentation with acrylamide chemistry and a hint of sulfonic acid engineering. The extra methyl branch alongside the sulfonic acid not only improved solubility but gave materials scientists plenty to work with for everything from water treatment to oilfield chemistry. Key patents in the 1960s reflected these explorations, and multi-ton commercial production took off over the next few decades, helping to unlock new frontiers in industrial water management, advanced textiles, and construction additives.
At a glance, AMPS looks like a simple white or off-white powder, but it's anything but ordinary. Chemists have come to treat it as an indispensable tool for tackling problems involving charge density, solubility in harsh conditions, and strong adhesive qualities. Its molecular structure features both amide and sulfonic acid groups, so it straddles two worlds—one rooted in hydrogen-bonding potential, the other thriving in extremes of pH, hard water, and solvent exposure. That’s why any warehouse holding this product probably runs through it quickly, especially in areas dealing with scaling, improved recovery of oil, or tricky co-polymer blends for paints and coatings.
Physically, AMPS usually appears as a free-flowing crystalline powder, though pellet and bead forms are available for high-volume applications. In water, the compound dissolves quite easily, even at high concentrations, producing clear, colorless solutions. Its chemical backbone gives it a high melting point—usually well above 185°C—and delivers impressive stability under acidic or alkaline conditions. The molecule resists hydrolysis, so it holds up during long-term storage or exposure to brines and aggressive reagents. The strong sulfonic acid group gives AMPS its signature: high ion-exchange capacity and a knack for maintaining function where lesser monomers break down.
Labeling and handling require detailed attention to purity and residual monomer content. Reputable suppliers specify AMPS at purity levels upwards of 98 percent, with moisture content kept below 0.5 percent to avoid unwanted clumping or slow dissolution. Common specifications list residual acrylamide far below accepted safety thresholds, since many end uses target drinking water or regulated environments. Standard packaging revolves around double-walled polyethylene-lined drums, ensuring stability in humid or temperature-variable warehouses. On labeling, clear CAS numbers and UN codes help logistics teams stay compliant, and the labeling often includes full traceability for quality audits and recalls.
AMPS production takes place through a well-honed synthetic route where acrylonitrile meets isobutylene sulfonic acid (or its sodium salt), followed by a careful hydrolysis under tightly controlled pH and temperature. Batch reactors designed for strong acid handling come into play; the process demands steam-stripping, centrifugal separation, and meticulous neutralization steps to reach the right molecular weight and reduce color bodies that could hamper downstream use. Filtration rigs and quality control checkpoints double-check the absence of heavy metals or unreacted acrylamide, since even tiny residuals upset sensitive polymerization reactions later.
The dual-functionality of the amide and sulfonic acid groups means AMPS layers easily into almost any free-radical initiated copolymer, whether paired with acrylic acid, acrylamide, or specialty vinyl compounds. Polymerization proceeds under nitrogen or argon with water as the main solvent. For specialty uses, chemists sometimes derivatize the nitrogen—or form crosslinks through the sulfonic groups—to tighten the mesh in gels or enhance membrane permeability. In industrial research, teams regularly modify AMPS-based polymers through sulfonation or amidation, tailoring properties for superabsorbent materials or advanced flocculants.
The IUPAC name rolls off the tongue as 2-Acrylamido-2-methyl-1-propanesulfonic acid. Over the years, industry has helped shorten this to AMPS or simply 2-AMPS. Trade names and regional monikers pop up in patents and global commerce, each reflecting subtle differences in grade or target application. Some manufacturers offer blends under names such as Aquatreat, Lubrizol’s AMPS, or KCI’s AMPS monomer. Each carries its own batch-to-batch documentation, but regardless of the trade dress, seasoned buyers know exactly what they’re getting.
Handling AMPS takes more than gloves and goggles. Its granular or powder form can irritate skin and respiratory tract. Manufacturers recommend respirators for large-scale charging, with local exhaust ventilation in dusty conditions. Since AMPS is non-volatile and stable at ambient temperatures, risks of runaway reactions look low, but the story changes if accidental mixing with strong oxidizers or concentrated alkalis occurs. In my years around pilot plants, emergency drills often considered the clumping issue, since wetted AMPS can form slippery floors and plug equipment. Disposal plans always emphasize minimizing water discharge, as high loads may impact local wastewater treatment if not neutralized.
Practical uses stretch across sectors. Water treatment plants favor AMPS polymers for making scale inhibitors and super-strong polyelectrolytes. Oilfield engineers blend it into drilling fluids and completion brines, where it controls formation damage and boosts oil recovery—especially under high salinity. Paint formulators rely on AMPS-based copolymers to increase scrub resistance and weatherability in exterior coatings. Textiles, adhesives, and even superabsorbent diapers get a boost from modified AMPS, the sulfonate group pulling moisture in and the amide sticking tough to fibers. Even in medicine, hydrogels based on this monomer turn up in wound dressings and controlled-release drug carriers.
Research labs continue to unlock new uses for AMPS by mixing it in next-generation copolymers or blending with nanomaterials. In the past five years, academic publications describe its role in more heat-resistant concrete for infrastructure, anti-scaling membranes for desalination, and durable coatings for solar panels. Researchers at universities in Europe and Asia keep testing AMPS-based systems for environmental remediation, especially for removing heavy metals from groundwater. Every time I visit industry trade shows, new patents pop up around AMPS block copolymers, and collaborative projects often reach out toward sustainability by swapping petroleum-based comonomers for biobased partners.
Toxicological profiles matter, especially with tightening regulations. Studies point out low acute toxicity for AMPS in common formats, but unreacted acrylamide still raises alarms in drinking water or food packaging applications. Regulatory bodies such as the EPA and EFSA keep looking for improved analytical methods to track AMPS residues, but the sulfonic acid and amide functional groups themselves show no evidence of bioaccumulation or carcinogenicity in repeated testing. Skin sensitization remains possible with extended, unprotected contact, but risk drops sharply with personal protective equipment, clean handling, and well-ventilated environments. Some companies support additional long-term studies to address occupational exposure and aquatic release, hoping to stay ahead of emerging guidelines.
Looking ahead, AMPS holds appeal as industry balances performance with tighter sustainability targets. As demand rises for biodegradable and renewable co-monomers, researchers juggle AMPS with green chemicals to build smarter, more responsible polymers. Construction and water management sectors, always in search of tougher, more reliable additives, see AMPS as a path toward longer lifespans and less frequent repairs. With climate-related weather swings battering infrastructure and pushing demand for advanced materials, the adaptability of this molecule makes it a permanent fixture in any lab or plant designing for tomorrow. What matters most—a relentless focus on innovation grounded in clear-eyed safety practices—keeps AMPS in the running every time new challenges emerge.
2-Acrylamido-2-methylpropane sulfonic acid, which people usually shorten to AMPS, finds its way into more corners of the industrial world than most realize. Picture the world of water treatment, where clean water matters more than most folks think about in a day. AMPS grabs attention in this field because it helps keep membranes clean. More than just an extra, this chemical forms a backbone for copolymers that stop scale from building up and block bacteria from getting comfortable. Water treatment plants rely on this to keep systems running and water sources flowing clean, not just in cities, but in food plants and paper factories.
Construction jobs often live and die by mixtures. Additives decide how concrete holds together under heavy weight day after day. AMPS delivers strength in this field. Builders add AMPS-based polymers to increase concrete strength and keep it from cracking when exposed to chemicals and changing weather. My friend in civil engineering always mentions these admixtures as the kind of behind-the-scenes fixers that most passersby never hear about. Skyscrapers and bridges end up stronger because of this one tweak.
Factories making textiles or paper deal with scale buildup more than most might realize. A shirt or a book page might look simple, but the water running through those big machines can get clogged up if minerals start sticking around. AMPS works in the fiber slurry, helping keep things moving and the final product looking right. Paper made more durable; fabrics take color evenly. That might not seem like a big deal until you notice a book page falling apart or clothes that come out faded after the first wash.
The oil field never shies from harsh conditions: salty water, heavy heat, and pressure. Drilling fluids built with AMPS-based polymers handle these extremes better than other chemicals that used to fill the same spot. These polymers stop calcium and other salts from jamming up the works. That means smoother drilling, fewer breakdowns, and bigger savings. I’ve heard from oil workers who appreciate anything that stops downtime—and AMPS draws fewer complaints than older ingredients in the muds and cement they pump underground.
As new rules tighten around chemical use, producers have to prove their products won’t create problems down the line. AMPS falls into a category that scientists can track and treat. Water treatment experts watch for proper breakdown and manage disposal byproducts. Some environmental scientists have raised flags about persistent chemicals in the past, so industry experts like to see that this compound doesn’t stick around long-term or accumulate in ways that lead to wider damage. Planning with sustainability at the center matters as more communities look to build water-smart and energy-efficient futures.
Industries lean on AMPS because the benefits roll through the supply chain: lower maintenance costs, less waste, and longer-lasting materials. But the push toward greener manufacturing creates pressure to keep improving these formulas. Some companies already blend AMPS with other ingredients to reduce impact, aiming to satisfy both engineers and environmental leads. The search for safer, just-as-effective additives keeps research teams moving, with AMPS usually right at the center of their work. I’ve seen change happen fastest on factory floors and in city operations, where one smart shift can ripple through hundreds of workplaces, showing that even old ingredients keep proving their worth by adapting to new demands.
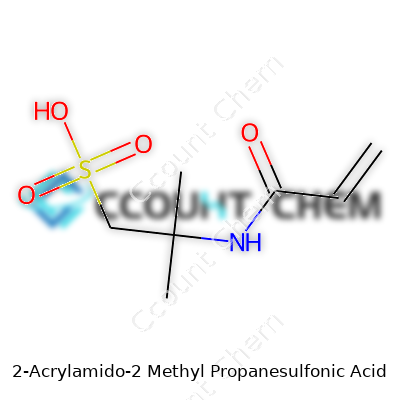
Anyone who’s worked at a bench knows the value of a compound that doesn’t throw surprises during synthesis. 2-Acrylamido-2-methylpropane sulfonic acid, or AMPS, brings that kind of reliability. Its structure includes both an acrylamide and a sulfonic acid group, each offering a useful function. The amide allows the molecule to join in with other monomers in polymerization, so you can get strong, stable chains out of it. That sulfonic acid group? It delivers a negative charge right where it counts, letting any polymer made with AMPS suck up water like a sponge and stay flexible.
Some chemicals fold under pressure. AMPS keeps working in tough spots. In oil recovery, water treatment, and textile dyeing, the job often brings heat, salt, and all kinds of pH swings. Polymers made with AMPS don’t lose their cool—literally or figuratively. During polymer testing, I’ve seen AMPS co-polymers keep their shape and function where cheaper acrylics fell apart. The secret sits in that bulging sulfonic acid group, which helps those molecules hang on to water and stay working even in briny or acidic solutions.
Real stories grow from everyday challenges. I watched a water treatment team spend weeks troubleshooting polymer breaks until a chemist tossed AMPS into the mix. Contaminants dropped, the solution stayed clear, and the plant cut back on chemical adjustments. The same holds true in grueling oilfield conditions, where brines and high pressure kill weaker formulas. Adding AMPS to polymer drilling fluids stretches lubrication windows and shrinks clogging.
It only takes a quick check of the periodic table to appreciate that a sulfonic acid group makes a molecule “hydrophilic.” In field work, this means polymers become better at swelling and holding on to water. That property saves a lot of time in designing super-absorbent products, grouts, and hydrogels. More than just water, the negative charge from AMPS stops other ions from interfering, so the end product stays efficient in salt-heavy scenarios like desalination or mining.
Wide use means more eyes on environmental safety. AMPS itself doesn’t build up in animals or the environment, but the sulfonic acid means it rinses out with water. Waste streams need monitoring, and teams must use proper containment. Chemists can trim waste with precise reaction control or switch to greener processing when possible. I’ve seen companies collect AMPS-laden wastewater for neutralization and reuse, cutting chemical spend and easing regulatory headaches.
AMPS isn’t just another additive. Its heat, acid, and salt stability opens up projects that would struggle with basic acrylates. The strong affinity for water and charge balance from that sulfonic acid group makes it a clear winner for anyone battling tough manufacturing or environmental conditions. Growing demand invites more innovation—and hands-on responsibility—in how we use, recover, and recycle this chemistry. With the right approach, AMPS isn’t just a building block; it’s a way forward for resilient applications in water, energy, and industry.
2-Acrylamido-2-methylpropane sulfonic acid, usually just called AMPS, shows up across a handful of industries. It helps keep concrete from setting up too soon, makes water treatment more efficient, and plays a part in improving oil drilling. Some folks see the complex name and assume hidden dangers. That’s always a fair question to raise, especially since we hear a lot about chemical hazards in the workplace.
People who spend time around industrial labs or manage chemical shipments always have their eyes peeled for red flags: cancer links, lasting burns, sneaky side effects. AMPS doesn’t lurk on most lists of notorious hazards. So far, the data shows it isn’t likely to trigger major, long-term health issues if handled with care. The warnings boil down to what you’d hear with many industrial chemicals—skin irritation, eye stinging, maybe some trouble if large amounts get inhaled as dust or dissolved in water.
Rubbing up against it or getting splashed can cause redness and discomfort, sort of like mild detergents or cleaning agents. The main toxicology research notes that AMPS is neither acutely toxic nor a carcinogen. No studies connect it to reproductive risks. It doesn’t pass through skin easily and doesn’t accumulate in the body over time. Swallowing a small amount by accident probably wouldn’t create a crisis, but it’d send most people to the sink to rinse their mouth out right away because of the awful taste and irritation.
I once toured a water treatment plant in mid-summer, tracing lines of powder and liquid chemicals from delivery to storage. Safety measures were plain—goggles, gloves, and lots of signage. Employees respected AMPS, but nobody seemed especially nervous. Their stories centered on split bags turning a morning into a cleanup job, not an ambulance call.
OSHA sets clear guidelines: keep things ventilated, suit up with gloves, and protect your eyes. These rules match what’s found on most AMPS safety data sheets. Even a few minutes digging into incident reports or regulatory records on AMPS reveals something important—it doesn’t show up on lists that track large numbers of workplace injuries or environmental spills.
There’s always the big-picture worry about chemicals—where does it all end up? AMPS mixes readily with water but isn’t persistent in soil or natural streams. Bacteria can break it down. Because it’s not classified as a hazardous waste and doesn’t threaten aquatic life at typical use levels, it stays low on environmental risk radars.
Big spills require cleanup and common sense, but small releases won’t unleash threats that hang around for years. Unlike some older chemicals, AMPS doesn’t stack up in food chains. Local regulators sometimes set thresholds, but these limits mostly reflect responsible practice, not crisis prevention.
Staying safe boils down to familiar moves for folks handling any lab or plant material. Keep AMPS away from bare skin and open eyes, avoid stirring up dust, store it securely, and label it clearly. Training that sticks with people—sharing stories about what happened on real days at work—beats robotic rule-following every time.
Better engineering controls, such as enclosed mixing stations and proper ventilation, keep risks down. Companies keeping good logs and letting workers report close calls create safer spaces for everyone. No need for alarm, but neither should it be seen as harmless. Everyday respect for chemicals pays off at every step, from unloading pallets to washing up at shift’s end.
I don’t think enough people realize that chemical names on a drum can mean daily headaches or health scares if ignored. 2-Acrylamido-2-Methylpropane Sulfonic Acid, often shortened to AMPS, falls into this category. It’s not flashy, but anyone working with polymers, water treatment, or oil recovery will run into it eventually. Industry values its chemical stability and solubility, but those same traits can make it tricky from a storage and handling viewpoint. The focus here is on real safety—not just following a checklist, but understanding what to actually do in a warehouse or lab.
AMPS is a solid or white crystalline powder. It isn’t explosive or radioactive. It's not a villain out of a disaster movie, but it brings enough skin and eye irritation to ruin a day. In the presence of moisture, it clumps up and starts to degrade, making it less effective for its main uses. Chemistry turns on you quickly once the packaging fails or water sneaks in. Stories from old labs are full of ruined batches just because someone left the bag open for a few hours.
I’ve watched teams try to cut costs on storage only to end up tossing half their materials. AMPS sits best in a cool and dry location, away from direct sunlight and sources of heat. Humidity makes the powder cake together and messes with its performance; a sealed container with an airtight lid beats a half-crumpled bag every time. Most industrial storage rooms set the thermostat between 15°C and 25°C. A good rule: the less temperature swings, the better your product.
Don’t stack heavy objects on top of bags or bins. Breaking a seal, even a little, invites moisture. I’ve seen small labs toss in silica gel packs to help, but a solid bucket with a screw cap gets the job done on a bigger scale. Clear labeling and separate storage from strong acids, bases, or oxidizers means fewer nasty surprises if a spill happens.
Anyone who handles AMPS daily should know personal protection—not just gloves, but goggles and long-sleeved clothing. Even seasoned technicians sometimes skip it “just this once,” and end up with red skin or sore eyes. The powder floats in air easily, so scoop smoothly to avoid creating dust clouds. If you have to move a lot, a simple dust mask catches most of the airborne particles.
For mixing, dump the powder slowly into water while stirring. Dumping it all in at once creates clumps that take forever to dissolve and can splash. A spill should never wait, especially if water is nearby. Use plenty of water for washing down and a dedicated chemical spill kit.
Ignoring storage rules seems easier in the short term, but it always backfires. Product loss, safety risks, and worker downtime cost much more than a proper shelf or container. Training goes a long way. People remember advice shared over coffee: keep it dry, use your gear, cap that bucket. Smart logistics means less stress, better results, and fewer trips to the emergency shower. That’s what earns trust from regulators, workers, and clients alike.
Plenty of problems get solved just by respecting the basics: dryness, cool conditions, clean containers, and personal protection. Putting those ideas into every shift turns a hazardous acid into just another part of the job. The routine protects the people, the product, and the bottom line.
Mixing chemicals to make better cement turns out to be less mysterious than it sounds. 2-Acrylamido-2-methylpropane sulfonic acid, known in labs as AMPS, often ends up in concrete mix for high-performance construction. I first noticed the difference in how superplasticizers based on AMPS worked when pouring slabs on hot summer days. The mix didn’t dry out as quickly, and the concrete flowed into every gap. That meant fewer cracks months down the road and longer-lasting highways and bridges. According to published research in Cement and Concrete Research, superplasticizers based on AMPS maintain strength in salty marine environments, which is a big deal for coastal cities fighting rebar corrosion.
Dirty water isn’t just a problem for factories; it affects everyone. Water treatment plants use AMPS-based polymers because these chemicals don’t lose their edge in challenging conditions. The municipal team in my city told me they picked sulfonic acid-based polymers for treating both sewage and storm water, especially in colder months when other chemicals struggled. Researchers found AMPS polymers stay soluble and effective even with temperature swings and varying salt content, which isn’t something every polymer can do.
Energy companies face tricky recipes in oilfields. Salt and heat usually make standard chemicals useless. Polymers built around AMPS hang tough where others break down, so they push out more petroleum during enhanced oil recovery. That turns marginal wells into productive assets, which keeps jobs afloat and stabilizes local economies. During a consulting stint, I saw field operators swear by AMPS copolymers to make drilling fluids that resist the thickening action of calcium and magnesium in underground reservoirs.
Papermakers keep an eye on the chemicals that help paper dry fast and keep dyes vibrant. AMPS-based copolymers show up in specialty paper, helping ink anchor to slick catalogs or glossy magazines. In textile plants, AMPS copolymers turn up in dye baths. They help colors spread evenly and prevent streaking, so clothes look brighter out of the box. During a visit to a textile mill, I watched operators troubleshoot blotchy dyeing. By swapping to an AMPS-containing dispersant, the color stuck properly to every batch, saving hours on rework.
Every chemical comes with safety hoops to jump through. AMPS’s performance is balanced by solid evidence for its safety profile. Regulatory bodies have weighed in, and its use in industrial settings follows strict guidelines. Factories invest in containment and monitoring because keeping workers and communities safe matters more than ever to both customers and regulators. Strict oversight keeps production moving while protecting people and the environment.
Better chemistry means getting to know problems up close. Industries that use AMPS look for ways to cut waste and improve product life. Collaboration across specialties—from civil engineers to plant operators—pushes forward smarter, safer uses for this sulfonic acid derivative. As more data rolls in, I expect to see greener processes and lower costs alongside applications we haven’t even dreamed up yet. History shows these kinds of tools don’t just fix industrial bottlenecks; they change daily life.
| Names | |
| Preferred IUPAC name | 2-methyl-2-[(prop-2-enamido)sulfonic acid]propanoic acid |
| Other names |
AMPS 2-Acrylamido-2-methyl-1-propanesulfonic acid 2-Acrylamido-2-methylpropanesulphonic acid α,2-methyl-2-propenoylamino propane sulfonic acid 2-Methyl-2-acrylamidopropane sulfonic acid |
| Pronunciation | /tuː əˌkrɪl.əˌmiːdoʊ tuː ˈmɛθ.əl proʊˌpeɪnˈsʌl.fɒnɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 15214-89-8 |
| Beilstein Reference | 1722465 |
| ChEBI | CHEBI:53499 |
| ChEMBL | CHEMBL77986 |
| ChemSpider | 13924 |
| DrugBank | DB03255 |
| ECHA InfoCard | 03d5fa4e-3f4c-459e-bb72-61d30946aa2f |
| EC Number | EC 213-876-6 |
| Gmelin Reference | 88936 |
| KEGG | C06470 |
| MeSH | D000186 |
| PubChem CID | 6916 |
| RTECS number | BRD5786900 |
| UNII | X0WU270PLB |
| UN number | UN2585 |
| CompTox Dashboard (EPA) | DTXSID6023451 |
| Properties | |
| Chemical formula | C7H13NO4S |
| Molar mass | 207.24 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.302 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.0 |
| Vapor pressure | 1.7 hPa (25 °C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 9.3 |
| Magnetic susceptibility (χ) | -6.13 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.510 |
| Viscosity | 10-15 cps (15% in H₂O, 25°C) |
| Dipole moment | 4.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1610 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye damage. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 2-3-2-W |
| Flash point | > 100 °C (212 °F) |
| Autoignition temperature | 445 °C |
| Lethal dose or concentration | LD50 Oral Rat 1950 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral > 2000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | PEL (Permissible) for 2-Acrylamido-2 Methyl Propanesulfonic Acid: Not established |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Acrylamide Methacrylic acid 2-Acrylamido-2-methylpropane sulfonamide Sodium 2-acrylamido-2-methylpropane sulfonate N,N-Dimethylacrylamide |