The roots of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid trace back to research in polymer and water treatment fields during the late 20th century. Chemists began looking for alternatives to plain acrylic acid, hoping for something that stayed soluble in water, copolymerized easily, and delivered tough resistance against salts and harsh chemical environments. Once the structure with the sulfonic acid group and the acrylamide backbone was discovered, labs all over the world latched onto the compound for water purification, superabsorbent materials, and anti-static agents. After a few years, manufacturing at scale started picking up, following a climb in demand in oil recovery, textiles, and municipal water systems.
2-Acrylamido-2-Methyl-1-Propanesulfonic Acid, also called AMPS, stands out for its stiff backbone and chemical persistence. As a monomer, it lends itself to forming tough copolymers, which slip right into water-based paints, paper coatings, and superadsorbent gels. AMPS makes it possible to add specific characteristics to polymers—including ion exchange and thickening—through its side chains. Many industries depend on AMPS-based copolymers to hit specs that otherwise seem out-of-reach with traditional monomers. This includes everything from scratch resistance in coatings to keeping cement fluid during pumping.
AMPS appears as a white crystalline powder, and draws water from the air quickly thanks to its sulfonic acid group. Its solubility in water sits near the top of the scale for small organic acids. I know from working in a research lab that storing this compound with excessive humidity triggers clumping, so always reach for low-moisture environments. It melts at about 185°C and maintains stability up to moderate processing temperatures. Chemically, the molecule's double bonds invite straightforward radical polymerization. Its sulfonic acid group confers acid strength, strong ionic character, and unique capabilities for interaction with metal ions or organic cations. This stands out during water treatment applications, where it can bind hard ions effectively and outlast many organic additives.
Manufacturers commonly deliver AMPS in purity grades above 99%, with a focus on keeping sodium, chloride, and other salts at minimum levels. Standard packaging includes high-density polyethylene bags or drums, which insulate the powder from ambient moisture. Labels specify exact lot numbers, expiration dates, primary hazards—like skin and eye irritation—and instructions for safe handling. Detailed specs usually call out moisture content, sodium content, and degree of polymerization where applicable. These details matter, especially in regulatory audits or when customers face strict compliance in food-contact or potable water applications.
The process for making AMPS starts with acrylonitrile, which undergoes hydrolysis and sulfonation in a carefully controlled reactor. This two-step transformation requires precise temperature and pH control. Early pilot plants often struggled with unwanted byproducts, especially if any oxygen crept in. Today’s operators keep tight control of air exposure, use inert gases, and monitor reaction dynamics through automated systems. The final product gets washed and filtered to strip out unreacted material, dried under vacuum, and ground to a fine powder. In my time shadowing a chemical plant operator, I learned a small shift in reaction pH often throws off the yield or purity, so it’s clear that technical expertise carries major weight here.
AMPS takes part in radical polymerization with vinyl monomers, delivering improved water solubility and salt resistance to the resulting polymers. Grafting reactions with polyacrylamide or polystyrene form the backbone of commercial water-soluble polymers. In the lab, chemists sometimes tweak the side chains, attaching hydrophobic groups or introducing functionality for molecular recognition. AMPS copolymers turn up in viscosity modifiers, scale inhibitors, and flocculants. For oil fields, copolymer blends usually include AMPS to keep viscosity in line during steam flooding or high-temperature injection, even in brine with elevated salt levels.
This acid goes by a long list of synonyms: 2-Acrylamido-2-methylpropane sulfonic acid, AMPS, and its sodium salt version (NaAMPS), among others. Chemical suppliers flag these names on specification sheets and labels, recognizing how often products cross borders. Patents and technical data sheets claim product names such as Aquatreat AMPS and Lubrizol® AMPS, marketing the monomer for use in industrial processes, paints, or specialty plastics.
AMPS falls in the category of chemicals that call for respect but not outright alarm. Direct contact with skin or eyes brings irritation, so chemical-resistant gloves and goggles are not optional in a production setting. Inhalation over long periods can irritate the respiratory tract. Production operators train to use engineering controls, such as ventilation hoods and dust capture systems. Spill kits handle accidental releases, and regulations set maximum allowable concentrations in workplace air. Disposal routes follow local hazardous waste rules, making sure the compound doesn't build up in water systems without full treatment.
Water treatment facilities rank high among AMPS users, counting on its polymers for flocculating and sediment removal. In oil and gas recovery, polymers based on this acid help keep drilling fluids at workable viscosities and prevent solid buildup in pipes. AMPS also takes a role in construction—polymers ensure smooth flow in cement and concrete, even in hot or salty environments. In paper mills, using AMPS-based products brings up retention rates for fillers and strengthens wet-end additives. Coatings and adhesives formulas use AMPS to increase water resistance and durability. Personal care products sometimes call on this acid in gel form, especially where water absorption and stability under tough chemical conditions matter.
Research teams around the globe keep turning up new ways to tweak and apply AMPS. Blending the acid with rare or unconventional monomers delivers performance leaps in water-blocking cables or eco-friendly consumer products. Efforts in controlled-release fertilizers, superabsorbent diapers, and drug delivery systems continue to pop up in journals and patent filings. Experience in the lab showed me the stubbornness of this molecule under harsh tests—high temperature, acid, or caustic baths rarely shift its performance. Research on green chemistry techniques includes swapping traditional solvents for water-based processes and reducing side-product generation. These improvements aim at reducing carbon footprint, aligning production with evolving environmental targets.
Toxicological data for AMPS reveals low oral and dermal toxicity in animal testing, which reassures users that limited workplace exposure poses manageable risks if controls work as expected. Aquatic toxicity studies tell a more complicated story—like many strong acids, AMPS impacts freshwater organisms at high concentrations, underscoring the importance of strict effluent treatment in industrial plants. Chronic exposure studies remain ongoing, especially regarding breakdown products and persistent residues in soil and waterways. Regulatory agencies in Europe, North America, and parts of Asia continue to review these findings, updating safety limits and handling recommendations where needed.
AMPS shows great promise across fields clamoring for strong, adaptable, and environmentally sound polymers. Growth in clean water technology, oil-free drilling lubes, and sustainable construction points to steady demand. Research on biodegradable derivatives and renewable-source alternatives adds momentum to the field. Industry forecasts predict expanded uptake in 3D printing resins, functional textiles, and biomedical hydrogels. As regulations tighten around microplastics and persistent environmental contaminants, AMPS-based chemistry faces higher hurdles, but also opens up new spaces for innovation. With its unyielding resistance to chemical abuse and heavy metals, AMPS sits locked in as an anchor for next-generation materials—not just for industry giants, but also for research teams pushing boundaries in sustainability and environmental protection.
2-Acrylamido-2-Methyl-1-Propanesulfonic Acid, often shortened to AMPS, gets called out for some pretty important things in chemistry-heavy industries. While its chemical name might sound intimidating, its uses touch on everything from treating water to maintaining style in your favorite hair gel.
Many water treatment plants use AMPS in their polymer blends. This acid-based chemical mixes with acrylamide to form copolymers, which help separate solids from liquids. Picture a wastewater plant trying to filter sludge and dirt. Polymers with AMPS act like magnets, clumping together tiny particles so machines can easily pull them out. Studies have shown that AMPS brings an extra level of stability, even when water conditions get tough with lots of minerals or shifting pH levels. With increasing pressure in cities to save money and reduce pollution, consistent and stable performance in these purification systems can’t be underrated.
Builders and textile manufacturers benefit quietly from AMPS. Cement workers use special polymers containing AMPS in grouts and mortars. The chemical keeps mixtures from cracking open as they dry, even in dry or salty places. Ask anyone who’s fixed a broken sidewalk in a city with rough winters—cracks come back so quickly if the mix can’t handle moisture swings. The use goes beyond construction. Fabric makers mix AMPS-based agents into dyeing baths, letting colors stick to synthetic fibers while resisting fading from washing or sunlight. The textile industry used to rely on harsher chemicals that left water behind murky and chemical-laden. Shifting to AMPS-based processes means factories can cut down on waste, streamline rinsing, and make stronger fabrics at the same time.
Many hair gels and lotions rely on polymers that use AMPS as a key ingredient. These products keep their hold under hot or humid weather thanks to the sulfonic acid part of the molecule. Without this, that tidy hairstyle melts away in an hour or skin creams might start to separate. For anyone with allergies, another upside is that AMPS-containing products show much lower risk of causing skin irritation compared to many older formulas. That’s why many popular brands have shifted toward this modern mixture. The result is smoother, long-lasting results without sticky residue.
Oil drilling crews face wild swings in pressure, salt, and temperature as they try to tap deep deposits. AMPS-based polymers help drilling fluids stay thick, so they carry rock fragments up from underground without gumming up machinery. This kind of stability can mean the difference between a safe operation and expensive downtime. The chemical structure of AMPS adds strength even with the high salt levels found in some of the world’s biggest energy fields. No surprise then, oil services companies invest so much in this stuff—it saves money, keeps systems running, and protects workers below ground.
Behind every practical use of AMPS, smarter chemical design means less waste, better safety, and stronger products. It’s easy to overlook a chemical like this when talking about modern infrastructure or consumer goods, but reliability often starts at the molecular level. After seeing concrete hold up better, water run cleaner, and personal products cause fewer allergic reactions, the argument for AMPS-based improvements feels pretty compelling.
2-Acrylamido-2-methyl-1-propanesulfonic acid, often called AMPS, has earned respect in several industries because its chemical makeup brings a blend of reactivity and toughness that folks working with polymers really appreciate. The structure of AMPS revolves around an acrylic backbone, with a standout feature: the sulfonic acid group sitting at the end of a short, branched chain. This specific arrangement gives AMPS some real bite in water-solubility and ionic strength, unlike many standard acrylic monomers.
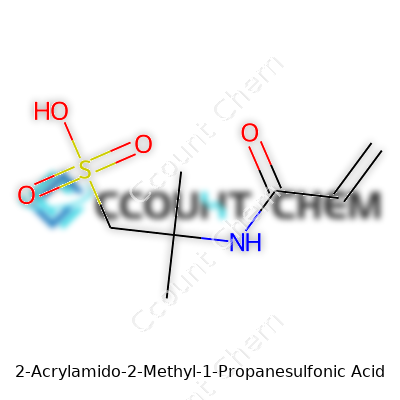
AMPS follows this formula: C7H13NO4S. Visualize the molecule as an acrylic group (CH2=CH–CO–) central to its reactivity, connected to an amide function (–NH–), with a methyl group branching out beside a sulfonic acid group (–SO3H). To map it out more directly, the full IUPAC name is 2-methyl-2-acrylamido-propane-1-sulfonic acid. In structure, it looks like this:
CH2=CH–CO–NH–C(CH3)2–CH2–SO3H
Every part of that structure serves a purpose. The acrylic double bond makes it jump into polymerization reactions. The amide group gives the resulting polymer toughness and resistance to heat. The sulfonic acid, which demands attention in water, brings in strong ionic character, helping finished products to survive in salt-heavy, harsh environments.
AMPS isn’t in demand just for the sake of novelty. In my own research back in chemistry labs, mixing acrylamide with AMPS always gave blends that outperformed simpler polymers. Everybody working in water treatment or making water-absorbing diapers knows how much pressure these materials face. Ordinary acrylics buckle under brine or heat. Add AMPS into the mix, and the sulfonic acid groups grab hold of water, keeping the polymer swollen and stable even when calcium and magnesium ions would shut down lesser products.
The specific structure does more than bond easily into growing chains. It can handle high pH, resist nasty microbes, and stay flexible in salty or hot conditions. So people in oil recovery, papermaking, and concrete admixture all lean on AMPS to get reliable action where it counts. Chemists figured out that not every acrylic needs to be bland or easily broken; a well-placed sulfonic acid steers the whole performance.
Problems facing industrial polymer applications often come down to chemistry that can’t withstand hostile environments. The AMPS structure, by blending a flexible acrylic backbone with a highly charged, hydrophilic group, brings polymers that not only last, but also adapt to different demands between water softeners and cement additives. Manufacturers can dial properties up or down, all starting from the same basic AMPS monomer, simply by tweaking the cross-linkers or the percentage of AMPS in a copolymer chain.
Better understanding of the chemical backbone—beyond just reading a formula—lets researchers design smarter materials, cut costs, and save time in trial-and-error blending. People in the trenches need reliable performance, and the right monomer structure, like that of AMPS, makes it possible.
In the world of specialty chemicals, 2-acrylamido-2-methyl-1-propanesulfonic acid—let's just call it AMPS for simplicity—is a mouthful and turns up mostly where folks are tinkering with polymers. It helps thicken up paints and detergents, gives concrete certain properties, and keeps industrial water clean. Now, anytime you see a name like this on a product label, worries start to bubble up—“Could this stuff be dangerous?”
If you handle AMPS powder, you’re going to want gloves and good ventilation. It can cause skin and eye irritation, which is not much of a surprise for people who’ve worked with chemicals for a while. A coworker once wiped his face with a gloved hand after handling something similar and ended up with red, itchy skin for two days. Eye exposure causes burning and watering. Inhaling dry powders is never fun; the dust can irritate your airways. So, for factory workers and researchers, the usual rule stands—protective eyewear, gloves, and dust masks are your friends.
Toxicity usually means “Will this chemical cause you more harm than a rash?” With AMPS, all the data I’ve dug up points toward low acute toxicity. It does not kill lab rats at reasonable doses, nor does it build up in tissues. Unlike notorious chemicals like acrylamide, AMPS skips the neurotoxicity. Swallowing big amounts—hardly likely outside a lab—gets you a stomach ache and maybe some nausea. No solid evidence for cancer risk pops up. The European Chemicals Agency, the United States Environmental Protection Agency, and several chemical safety boards all put AMPS in a low-to-moderate risk category—if you’re using proper safety gear.
The research gets thin here. Most people don’t keep getting exposed over years. Animal tests haven’t shown it builds up in the body, or messes with genes and DNA. But science only goes as far as the testing. That does not mean “ignore the risk.” Just don’t freak out if you see it in a product ingredient list—especially since AMPS mostly hangs around in finished, cured polymers that don’t escape into the environment or get absorbed through the skin.
Let’s be real—almost any chemical can turn nasty if spilled in large amounts or handled carelessly. AMPS dissolves easily in water, so it’d move through soil if large amounts were dumped outside, possibly affecting aquatic life. I’ve seen water samples downstream of industrial sites get cloudy, though low concentrations of AMPS don’t seem to wipe out fish or bugs. Wastewater treatment plants generally break it down before water heads back into rivers.
Industry training and worker protection needs ongoing attention. Labeling on packages ought to include clear instructions, not just “Caution” in small print. Jobs involving open-handling—mixing, pouring, or accidental spills—should always supply proper gloves and goggles, no exceptions. As a consumer, you probably never touch AMPS straight up, but manufacturers need to keep green chemistry in mind, searching for additives that are gentler on people and planet.
Chemical safety comes down to how people use a substance, not just what’s on the label. By keeping gloves on, goggles snug, and by staying informed, accidents just don’t happen as often. With AMPS, the risk stays pretty low if you respect it—just like any industrial chemical should be.
For those who work with chemicals, 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid (AMPS) stands out as one that rewards respect and preparation. This white, solid acid powder helps with water-solubility and copolymerization in industries like water treatment or oil extraction. Even though it’s valuable, ignoring safety measures can land a lab, warehouse, or production crew in a tough spot. Strong acids have a way of biting back when handled carelessly.
AMPS thrives in a cool, dry place—think consistent temperature, no risk of heat, sparks, or flame. Humidity doesn’t just clump it up: it may affect shelf-life and purity. Tossing it on a dusty warehouse shelf won’t cut it. I’ve seen humidity spike in storage rooms after a rainstorm, and fine powders can turn into sticky messes. Tight containers—sealed after every use—act as your front line of defense. After years of working with reactive powders, I’ve learned that air-tight, chemical-resistant containers keep you from costly product losses and accidental reactions.
Acids dislike basic substances and don’t tend to play well with oxidizers. If you ever forget this, an unplanned reaction can remind you. Store AMPS away from anything with incompatible reactants. In busy facilities, even a single misplaced container has ruined batches of expensive materials.
Before opening a bag or drum, double-check your PPE. Lab coats, nitrile gloves, chemical splash goggles, a mask for airborne dust—these save skin, eyes, and lungs from harsh irritation. I’ve seen even seasoned chemists skip a step during setup, only to deal with raw, burning hands later.
Good handling practices rely on organized spaces. Poor labeling or cluttered shelves nearly always go hand-in-hand with accidents. Supervisors and frontline workers both benefit from using clear, large labels, and following well-worn protocols. When transferring AMPS between containers, avoid spills with dedicated scoops and avoid cross-contamination by working with clean equipment every time.
Static can become a hidden hazard. Handling powders creates static electricity that may ignite air-dispersed dust. Ground equipment and avoid wool, polyester, or other high-static fabrics. In my first year on the job, a friend learned this the hard—or shocking—way, leading to an evacuation.
Ventilated storage spaces pull double duty: they prevent fume build-up and clear any accidental dust. A good exhaust system reduces both short-term exposure and long-term wear on facility infrastructure.
Spills happen, even with the best planning. Dry AMPS is easy to sweep if it’s fresh, but wet residue takes more elbow grease and may damage floors if ignored. Neutralize spills with lots of water, using non-reactive mops or cloths, and always double-bag contaminated waste for disposal. The key is not letting one mistake snowball into lingering contamination.
Turnover, new chemists, or even a new shift manager bring fresh hands to the job, so regular training is non-negotiable. Regular review of storage areas stops creeping neglect. Having a strong maintenance schedule for checking container seals, exhaust fans, and emergency eyewash stations isn’t about bureaucracy—it’s about building a trustworthy environment.
AMPS reminds us that safety grows from attention to detail, not fancy tech or “gut feelings.” Take care in storage and handling, and this useful acid stays in its place—serving its purpose, not causing problems.
Anyone dealing with chemicals learns quickly that packaging isn’t just an afterthought. It impacts safety, shelf life, usability, transportation costs, and even regulatory headaches. Take 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid (AMPS). This compound serves a starring role in water treatment, paints, adhesives, construction additives, and oilfields. Because it travels such a broad path, packaging becomes more than an exercise in convenience—it’s a non-negotiable point on safety and practical use.
Most chemical suppliers offer AMPS in powdered or granular form. Big bags line warehouse shelves—think 25-kg kraft paper sacks with a polyethylene liner for moisture resistance. Pallets stretch-wrapped for stability, labels visible for handling. These sacks allow workers to pour out as much as they need, reseal with a clip, and avoid major spills. I’ve seen chemical storerooms where broken bags meant slippery, hazardous floors. Small changes—such as inner liners or double-bagging—cut down on these accidents and keep cleanup simple.
Larger users—textile factories or water treatment plants—skip sacks and order AMPS by the drum, commonly in 25 or 50 kg sizes, or even super sacks at one metric ton. Rigid, high-density polyethylene drums fend off humidity and rough treatment. Some plants build in larger bulk storage, so they opt for big bags (1000 kg) lifted by forklift, which keep handling and waste down. Bulk delivery arrives in tanker trucks, sometimes with AMPS dissolved for immediate use. That approach reduces dust exposure and speeds up manufacturing lines—I saw it in action in a busy paint plant. Instead of half a dozen workers handling bags, a single operator manages a hose and controls the flow from truck to storage tank. The workplace stays cleaner and workers face fewer risks of skin or eye contact.
Hydroscopic chemicals like AMPS don’t play nice with moisture, so strong packaging makes a difference. The manufacturers who get it right seal every bag at the seam, line them with plastic, and sometimes even include desiccant packets for longer shipments. Cost-cutting here leads straight to losses, as caked or pre-reacted product needs tossing. I’ve been involved in product audits triggered by clumpy powder—someone down the supply chain decided to skimp on the packaging, and the whole batch failed water solubility tests. It wasted time for everyone involved, from truck drivers to plant managers.
Labels add another wrinkle. Chemical packaging requires legible labels showing batch codes, hazard signs, country of origin, and handling instructions. This isn’t just red tape—clear labeling can prevent accidents, especially during quick shifts or contractor handovers. When workers spot those pictograms and warnings, it gives them a fighting chance to pull gloves or a mask from their kit before anything goes wrong.
Many manufacturers see opportunity in reducing single-use plastics and improving reusability. Manufacturers and users meet at industry conferences, swapping ideas about reusable drums or collapsible bulk containers. Reliable closed systems—sealed connections between delivery truck and storage tanks—minimize both emissions and waste. I once worked with a team testing smart containers that ping suppliers when stocks run low, cutting unnecessary storage and road trips. These small victories build over time: less waste, fewer spills, lower costs, fewer headaches for everyone from the shop floor to the boardroom.
The move away from basic bags toward tougher, branded, trackable containers not only meets compliance but builds a sense of pride and trust in the supply chain. If chemical suppliers keep up this pragmatic mindset, safer workplaces and lower environmental impact will follow naturally.
| Names | |
| Preferred IUPAC name | 2-methyl-2-[(prop-2-enoyl)amino]propane-1-sulfonic acid |
| Other names |
AMPS 2-acrylamido-2-methylpropane sulfonic acid 2-acrylamido-2-methyl-1-propanesulfonic acid 2-methyl-2-acrylamidopropane sulfonic acid |
| Pronunciation | /tuː əˌkrɪl.əˈmæmɪdoʊ tuː ˈmɛθ.əl waɪn proʊˈpeɪn.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 15214-89-8 |
| 3D model (JSmol) | `3d:JSmol?modelid=AMPS` |
| Beilstein Reference | 1742547 |
| ChEBI | CHEBI:53499 |
| ChEMBL | CHEMBL418077 |
| ChemSpider | 21531 |
| DrugBank | DB16352 |
| ECHA InfoCard | 57-453-7 |
| EC Number | 381-060-8 |
| Gmelin Reference | 79090 |
| KEGG | C06360 |
| MeSH | D000196 |
| PubChem CID | 71199 |
| RTECS number | AS3326000 |
| UNII | 53R84EK50K |
| UN number | UN2585 |
| Properties | |
| Chemical formula | C7H13NO4S |
| Molar mass | 207.24 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.22 g/cm³ |
| Solubility in water | 2320 g/L (20 °C) |
| log P | -2.0 |
| Vapor pressure | 2.3 hPa (25 °C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 8.8 (Basicity of AMPS, pKb) |
| Magnetic susceptibility (χ) | -71.8×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.508 |
| Viscosity | 10-15 cps (15% in H2O, 25°C) |
| Dipole moment | 6.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid is 240.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1160.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2066 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye damage. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H314, H317 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P362+P364, P403+P233, P405, P501 |
| Flash point | > 100 °C |
| Lethal dose or concentration | LD50 Oral Rat: 1950 mg/kg |
| LD50 (median dose) | 1310 mg/kg (rat, oral) |
| NIOSH | GV7210000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | No REL established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Acrylamide Methacrylic acid 2-Acrylamido-2-methylpropane Sodium 2-acrylamido-2-methyl-1-propanesulfonate 2-Methyl-2-propene-1-sulfonic acid |