The roots of 2,5-diaminobenzenesulphonic acid wind back over a century, finding their place early in the annals of synthetic dyestuffs. Industrial chemistry began unlocking new colors for textiles and papers once this compound arrived on the scene. German and British researchers, chasing the demands of textile mills and emerging colorfast technologies, tweaked sulfanilic acids and related aromatic amines. The routines for sulfonation and amination did not just pop out perfectly. Consistent supply of starting materials and control over temperature changed the scale and safety of commercial operations. Today, we don’t worry about glassware breaking apart from variable heat like our chemical ancestors. Large reactors and digital controls step in, offering reproducibility chemists from the late 19th century would not recognize. Historical patents and academic publications continue to shape tweaks in industrial practice.
Chemists and manufacturers reach for 2,5-diaminobenzenesulphonic acid as a key intermediate in the synthesis of azo dyes and pigments. Its particular structure—an aromatic ring with two amino groups and a sulfonic acid—opens up countless chemical routes that find their way into many industries. Some consumers see the end result in vivid colors from printed materials or in the appearance of lovingly restored textiles. Manufacturing plants use it for coupling reactions and as a builder block for further functionalized compounds. Ease of purification and availability across most chemical supply networks keep it accessible for both research and production-scale projects.
This aromatic compound shows up as a crystalline solid, usually taking on a faint color somewhere between off-white and dull beige. Solubility in water comes from the sulfonic acid group, and once dissolved, it gives a faintly acidic solution. Two amino groups sitting on the benzene ring boost its reactivity during diazotization and substitution reactions, making it an attractive compound for those looking to modify molecules. The compound melts at a noticeably higher point due to strong interactions from sulfonic and amino substituents. Chemists watch for slight degradation under strong sunlight or at elevated pH—useful to know where long-term stability in storage and application is required.
Suppliers and users work with strict labeling protocols to match regulations from REACH and local authorities. Labels display the CAS number, molar mass, purity grade, and batch information. Commercial shipments often deliver the acid at a minimum purity of 98.5 percent, with moisture content below 1 percent and no scent of decomposition. Drums or bags used for shipping usually keep out excess light and moisture. Material Safety Data Sheets land next to each purchase, providing structure diagrams, handling guidelines, and disposal standards. These guarantees matter just as much to a university lab as to a scale-up pilot plant. Proper labeling does more than tick a checklist; it builds clarity for workers tasked with record-keeping or emergency response, reducing anxiety and mistakes.
Chemical manufacturers create 2,5-diaminobenzenesulphonic acid via sulfonation of phenylenediamine or by selective reduction of nitrobenzenesulfonic acid derivatives. The route often kicks off with nitration followed by catalytic hydrogenation or another reduction. Each step invites risks: uncontrolled temperatures can spawn thermal runaways, while incomplete reactions leave behind by-products. Modern industry uses continuous-flow reactors and fine-tuned temperature sensors to manage risk and maximize yield. A good operator tracks color changes and pH readings, not just the numbers fed to analytical devices. Waste streams from acidification or reduction need careful treatment to comply with environmental laws. While old bench techniques still benefit small batch work, anyone seeking consistent kilos or tons heads straight to optimized industrial synthesis.
The functional groups offer a reliable playground for further chemistry. Both amino groups react swiftly under diazotization conditions, sending azobenzene dyes or specialty pharmaceuticals down the pipeline. The sulfonic acid sets it apart from unsubstituted diamines—water solubility and further reactions with formaldehyde or other aldehydes. Chemists tweak these functional groups to lift reactivity, adjust solubility, or tie on longer specialty side chains. Details from kinetic studies and mechanistic insights shape how industry professionals time and temper each conversion. What sounds like a routine coupling or nucleophilic substitution actually demands close observation. The compound’s amphoteric nature lets it act as both acid and base, which drops surprises for anyone not double-checking pH or stoichiometry.
Language can get confusing with specialty chemicals like this. 2,5-diaminobenzenesulphonic acid also shows up as 2,5-Benzenediamine-1-sulfonic acid, Sulfanilic acid diamine, or its registry as C6H8N2O3S. CAS 4399-00-4 appears in most safety and technical listings. International traders might group this compound with other aromatic amines or use commercial nicknames from dye companies—none of which change the actual chemical, just the paperwork it travels with. Scientific publications often abbreviate or use synonyms; getting both the structure and traditional names right keeps confusion to a minimum and research reproducible.
Handling aromatic amines is serious business. Gloves, goggles, and fume hoods remain a daily fixture, not an afterthought. Prolonged skin contact raises sensitization concerns, especially in environments where workers prepare solutions or measure out solid samples. Chronic inhalation remains a documented hazard, and local exhaust systems cut airborne risks. Plant operators and bench chemists pay attention to spills because 2,5-diaminobenzenesulphonic acid’s water solubility means quick migration unless cleaned up immediately. Storage depends on sealed containers, low humidity, and minimal heat. Compliance with REACH, OSHA, and local chemical regulations relieves some burden of worry, but workplace culture built around routine safety drills and instant access to fresh gloves and solvent-cleaners makes a bigger difference.
Dye and pigment makers gave this acid an early head start, and that legacy continues. Its structure unlocks useful routes to reds, oranges, and browns in the world of synthetic textiles and paper dyes. Biochemists use it as a building block for enzyme substrates and stain-forming reagents. Environmental labs test its derivatives for water quality and trace contamination. The pharmaceutical sector values it for sulfonamide-based drugs and precursors. Research teams working on emerging polymers experiment with modified forms for electronic inks or functional coatings that resist fading. I’ve seen quality-control technicians in small labs lean on this particular acid as a reference standard, checking the calibration of their equipment with its reliable chemical signature.
The drive to push this compound forward has not stopped. Modern synthetic chemistry joins hands with green chemistry principles to develop safer, lower-waste syntheses. Companies sponsor research into replacing hazardous solvents and optimizing energy use. The academic community runs regular screens for new analytical methods to detect trace impurities, since some applications call for nearly total purity. New catalytic methods and bio-inspired reduction pathways might soon take over from classic tin or iron reductions, cutting down both cost and environmental impact. Research labs also look at the compound’s interactions in biological systems, tracking metabolic products in living cells or environmental samples. With every tweak or new discovery, we see broader applicability, fewer waste streams, and less risk for workers and communities.
Toxicologists approach aromatic amines with caution. Animal studies reveal possible links with liver and kidney stress after chronic exposure. Water solubility frees it to move quickly through soil and water, prompting toxicology testing across aquatic and terrestrial systems. Some studies point to moderate mutagenic risk under certain metabolic pathways, making safe handling a priority. Research continues into breakdown products formed during water treatment or combustion. Regulatory agencies scour published results before updating handling rules and acceptable exposure levels. Laboratories and factories tune their workplace hygiene and environmental controls based on these findings, using closed-system handling or boosted personal protective equipment. Continued funding and transparency in sharing negative results make a measurable impact on industry and regulatory confidence.
The growing call for more sustainable manufacturing sets the next stage. Demand for greener synthesis, cheaper catalysts, and higher yield steers current research and private sector investment. Policy makers and company strategists debate how to balance innovation with new safety data as more uses emerge. Emerging applications, such as advanced functional coatings and next-generation pigments durable to UV and ozone, rely on tweaks to the sulfonic acid core. As regulations shift and supply chains evolve, factories aim for cleaner, more efficient operations. Researchers and practitioners, sharing lessons from both industrial chemistry and frontline laboratory work, will keep adapting and plugging knowledge gaps. Over time, that steady progress shifts both chemical practice and public perception toward a more responsible, informed use of specialty ingredients.
Step into any shop selling colorful clothes, food packaging, or household cleaning products, and somewhere in the mix, you’ll find chemical ingredients pulling more weight than most people realize. 2,5-Diaminobenzenesulphonic acid is one such workhorse. If you’ve ever admired the rich hues in a new shirt or noticed the clarity in bottled soft drinks, you’ve likely crossed paths with its handiwork.
At the core, this compound serves as a building block in the production of azo dyes. Azo dyes show up in textiles, plastics, and even artistic inks. Many bright reds, yellows, and oranges in the fabric industry owe their vibrancy to reactions using 2,5-diaminobenzenesulphonic acid. Over the years, I’ve visited dye manufacturing setups and watched entire vats of color spring to life thanks to chemical reactions involving sulfonic acids. The smell, the heat, the way a pale liquid will burst into a familiar shade you’ll see on a street in Mumbai or a boutique in Milan — all point back to compounds like this.
Chemistry gives creative power, but safety can never take a backseat. The textile industry has faced pressure to phase out substances linked to environmental damage or health risks. Regulators in Europe and North America, for example, look hard at aromatic amines because of their potential health effects, especially if mismanaged. Scientific studies point to links between some aromatic amines and cancer, so responsible supply chain management matters.
I remember talking with a packaging engineer who explained how small tweaks in chemical recipes complicated supply chains, often for good reason. Sulfonated aromatic amines like this one help make optical brighteners — the stuff that makes paper products look crisp and white instead of yellow. Nobody buys a carton of eggs for the whiteness of its label, but clear, attractive packaging does influence choices at the grocery store. In addition, strict food safety rules govern any chemical used in materials touching food. I’ve seen paper producers scramble to switch suppliers or wrangle documentation after ingredient lists come under review.
I once sat in a meeting with environmental auditors discussing persistent organic pollutants. As more people take interest in what goes into their clothes, food wrappers, and even cleaning agents, industries face real pressure to use substances with cleaner safety and environmental profiles. For 2,5-diaminobenzenesulphonic acid, tracking exactly how it’s used, and where it ends up, requires discipline and investment. Some chemical companies now tout supply traceability as their selling point.
People working in chemistry sometimes get frustrated by red tape. They also know these rules come with good reason, especially when safer alternatives exist. Some manufacturers turn to bio-based chemicals, or look for less hazardous cousins in the aromatic amine family. Newer dye molecules sometimes promise lower environmental risk, often because they break down more easily or don’t release toxic breakdown products.
We’ve all got a stake in what gets used behind the scenes. For me, the most progress comes from practical steps: auditing supply chains, choosing less persistent chemicals, checking where runoff could pollute rivers. 2,5-Diaminobenzenesulphonic acid plays a critical part in bringing color and brightness to daily life, but accountability and innovation will decide if future generations benefit without paying unexpected costs.
2,5-Diaminobenzenesulphonic acid hits the senses as a solid with a signature crystalline texture. This stuff is water-soluble and draws in moisture from the air fast — a lesson every lab worker learns the hard way after a broken seal or careless handling. The structure puts two amino groups and a sulfonic acid group onto the benzene ring, making it punchy for both chemistry projects and some industrial jobs.
Drop this compound into water, and it dissolves quickly. That means storage matters — left out, it clumps and the purity slides as it pulls in moisture.
One thing I’ve seen over and over is how well this compound reacts with other stuff. The amino groups give it strong basic qualities, so it latches onto acids, especially in dye-making. It jumps into diazotization and coupling reactions — classic moves you’ll spot in labs feeding the dye and pigment industry. Those reactions don’t just transform the lab scene; they’ve shaped entire color palettes for textiles.
The sulfonic acid group, meanwhile, hands it strong acidity and makes the molecule highly polar. Water loves this compound, but it shies away from nonpolar solvents. This polar nature is what helps it mix smoothly in water-based applications. Years working with water-soluble dyes showed me the direct link between a chemical’s structure and the ease of cleanup — less residue, faster rinsing.
Put heat to it, and it decomposes, giving off some nasty fumes including nitrogen oxides and sulfur oxides. Safety in the lab or on the production floor isn’t negotiable — fume hoods and solid training protect both people and product quality. Mistakes cost more than money; I’ve seen colleagues knocked out of commission for days after ignoring solid handling rules.
Because 2,5-diaminobenzenesulphonic acid grabs onto other chemicals so well, it’s become a workhorse for textile dyes and pharmaceuticals. Its reactivity shrinks the steps needed in making azo dyes, which wind up staining clothes or food. Production depends on high purity, and even a small bit of contamination impacts the end color. Labs focusing on quality control keep a close eye on moisture level and impurities.
Safety data puts the spotlight on its irritant qualities, especially for eyes and skin. Once, someone on my team neglected gloves during cleanup — the skin rash was an ugly reminder. Companies must invest in personal protective gear for every level of worker. Waste treatment matters too. With sulfonic acid involved, untreated runoff threatens waterways, so dedicated neutralization systems become a non-negotiable cost of doing business.
As the industry leans into sustainability, reusable packaging and better containment for such reactive chemicals are gaining attention. Keeping it dry, cool, and tightly sealed limits unnecessary waste and cuts down on product loss. Real ingenuity shows up in waste stream management, capturing potentially hazardous byproducts before they ever leave the plant.
Looking ahead, green chemistry methods could mean greener synthesis, with milder conditions and fewer polluting byproducts. Switching to renewable feedstocks might cut back on emissions and waste. For now, strict controls and steady monitoring offer the most reliable shield against health and environmental fallout. Listening to workers on the floor — the people who see up-close what’s working and what isn't — remains the best way to drive safety and efficiency forward.
Working with chemicals like 2,5-Diaminobenzenesulphonic Acid brings a real responsibility. This isn’t just about wearing a lab coat and gloves; it means thinking about health and safety every step of the way. The dust from this compound can irritate the skin, eyes, and respiratory tract. When mixed with the wrong stuff or exposed to moisture, the risks go up. These aren’t far-off problems—they’re real concerns for anyone in labs or shops using dyes, polymers, or pharmaceuticals.
Space matters. Keep this acid in a cool, dry, well-ventilated area far from incompatible materials such as oxidizers and strong bases. Moisture and high heat can wreck the chemical’s integrity. Old shelving, cracked containers, and open bins invite trouble. I once saw a bucket left propped up near a leaky window, and within weeks, clumps and odd smells showed those safety shortcuts weren’t worth it.
Label containers clearly—don’t just rely on color coding or guesswork. Not every workplace is the same, so check that labels withstand splashes and won’t rub off after repeated handling. Place the original container inside a secondary tray to catch leaks, and schedule regular inspections. Lock storage rooms after hours, too. Security still counts, especially in shared buildings.
Don’t bring food or drink anywhere near this acid. It sounds obvious, but snacks tucked in a lab drawer or half-empty coffee mugs by the workbench just make contamination easier. Eating in chemical storage areas seems wild, but it happens—trust me.
Wear splash-resistant goggles and gloves rated for chemical work. Thin latex gloves won’t cut it if there’s a spill. If grinding, measuring, or filling hoppers, put on a fitted mask or work within a fume hood. Sudden splashes or powder clouds always catch the person who’s “just grabbing something quick.”
Train every employee, intern, and visitor who may touch or come into contact with this substance. Handing out a dated safety sheet won’t work if no one bothers to read it, so regular, hands-on training takes priority. Hold drills for spill response where teams learn to act rather than freeze.
Label safety showers and eye-wash stations so anyone can find them fast. In my experience, clear markings and unobstructed paths save precious seconds in emergencies. Don’t use these stations for regular cleaning or storage—keep them open and checked.
Follow local hazardous waste rules strictly. Don’t flush residues or rinse water down the drain; collect everything in marked, sealed containers. If your site doesn’t have professional chemical waste pickup, contact specialists for removal. Small spills need absorbent materials, sealed bags, and careful surface cleaning.
Safe chemical storage and handling come from more than procedures—they depend on habits. Setting up routines, double-checking supplies, and encouraging questions all matter. Involving the full team in safety walks and regular updates builds trust and keeps dangerous surprises to a minimum.
2,5-Diaminobenzenesulphonic acid isn’t something you find at the grocery store. It turns up in labs, sometimes in the manufacturing of dyes and specialty chemicals. Most people never hear its name. Yet, hidden in these technical corners, it’s got people asking if it’s safe to handle or if it causes harm to health or the environment.
I’ve learned to read safety data sheets before working with any chemical. For this compound, reputable databases like PubChem clarify its properties: it’s a crystalline powder, slightly soluble in water, and tends not to catch fire easily. Most concerning facts point to the skin and eyes. Splashes sting. Breathing in its dust isn’t ideal. The European Chemicals Agency lists it under “irritant” for skin and serious eye damage. But there’s no clear evidence that exposure leads to cancer, DNA changes, or birth defects. Chronic effects take years to study, so the science here remains open-ended.
Ask anyone handling any chemical in a plant or lab—if it burns your eyes, it gets respect. I’ve seen coworkers ignore eye protection around “low toxicity” compounds before, only to spend the afternoon blinking painfully. Experience teaches that even irritants matter if safety steps lag. Sulphonic acids like this one add another layer: their acidity brings sharp reactions on skin or with other substances. Wearing gloves and goggles stops most trouble before it starts.
Local water treatment techs know which chemicals mess up microbes or fish. This compound’s not high on most red lists, but it’s not totally friendly either. The sulfonic acid group means it stays dissolved rather than clumping up in water. If a batch leaks into waste streams and reaches a river, aquatic life might feel the effects. Certain studies note toxicity to small water crustaceans at elevated levels. In real-world situations, dilution reduces concentrated harm, but that’s no excuse to skip proper disposal.
Some labs and dye factories in developing countries run with little oversight. It’s a problem worldwide—workers exposed to unlabelled drums, no gloves, and no ventilation. Regulations like REACH in Europe set an example: the material shouldn’t stay in open air or wash down drains. Every workplace can make safety part of routine: clear labeling, personal protection, basic training. I’ve seen productivity drop fast in labs after cases of eye burns or skin rashes, far outweighing the few minutes it costs to use gear or check labels.
The truth is, every chemical brings some risk; it's the controls and respect for procedure that keep problems small. Switching to less hazardous alternatives, if available, can be one choice. Where I’ve worked, using splash guards, sealed transfer systems, and simple exhaust fans made life much easier. For smaller operations without resources for advanced equipment, basic steps like sealed containers and written instructions cut hazards sharply. Strong local policy enforcement keeps companies honest.
At the end, common sense backed by real data leads to fewer incidents and healthier workspaces. It’s up to industry and workers to stay educated, follow good habits, and respect the reality that a “minor irritant” can make a big mess if ignored.
2,5-Diaminobenzenesulphonic acid isn’t a catchy name you’d throw around at a dinner party, but in chemical labs and dye houses, people know its worth. You’ll see chemists write it out as C6H8N2O3S. Its official key, the CAS number, sits at 137-50-8. These figures allow researchers and industrial workers to buy, study, and refer to this compound without mix-ups—which can matter more than most realize, especially when one slip can lead to wasted resources or mixed-up research data.
Real-world use gives meaning to chemicals. 2,5-Diaminobenzenesulphonic acid has carved a niche in the synthetic dye industry. Walk down the textile aisles, and you find colors born from complex chemical blends, many of which depend on consistency and reactivity. This compound acts as a precursor or intermediate for crafting azo dyes—dyes that provide lasting, vivid shades to fabrics, leather, and sometimes food. Without precise chemicals like this, businesses struggle with faded clothes, inconsistent batches, or worse, unsold stock.
From my time observing production in a textile factory, I remember the commotion over a shipment of raw materials that didn’t meet specifications. It wasn’t just a missing batch code or an off-color powder; pure identification and traceability made the difference between a successful dye run and thousands of meters of ruined fabric. Employees checked and double-checked the CAS number—137-50-8—knowing a mismatch could mean days of lost work. Precision isn’t just science; it’s job security and business viability.
It’s easy to overlook the risks tied to handling aromatic amines. This compound, like many in its category, comes with its own hazards. Safety data sheets repeat the same warnings: skin and eye irritation, respiratory discomfort, and environmental stress if handled carelessly. Industrial hygiene depends on straightforward identification—both molecular formula and CAS act as the 'license plate' for safe transport and storage. Lab techs and chemical handlers need both the knowledge and the discipline to follow protocols. Health, and sometimes legal compliance, hinges on it.
You don’t need to look far to find a case of accidental exposure or improper waste disposal. Several cities have faced regulatory scrutiny after improper storage caused contaminated water supplies. Chemicals entering waterways can disrupt entire ecosystems. Responsible companies adopt industry best practices—not just to check boxes, but because their reputations and the trust of their workforce are at stake.
Traceable identity through CAS numbers and formulas could use broader adoption outside the lab. Governments and industry groups can push for digital tracking systems. Barcode scanning at each step of procurement, production, and distribution would make chemistry just a little safer—one scan can confirm the right chemical is in the right place.
Molecular precision, day-to-day habits, and clear documentation help chemical workers and the public alike. Real accountability comes from marrying science with practice—more than just memorizing a formula or number, but living up to the responsibility those numbers represent. That’s what builds trust in everything from dyes in your shirt to the research pushing medicine forward.
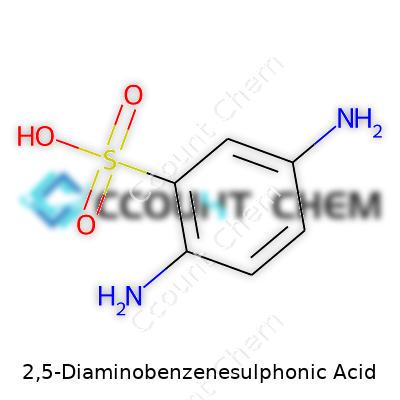

| Names | |
| Preferred IUPAC name | 3,6-Diaminobenzene-1-sulfonic acid |
| Other names |
2,5-Diaminobenzene-1-sulfonic acid Sulfanilic acid-o-phenylenediamine 1-Sulfo-2,5-diaminobenzene 2,5-Diamino-1-benzenesulfonic acid |
| Pronunciation | /tuː,faɪv daɪˈæmɪnoʊˈbɛnziːnˌsʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 88-68-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2,5-Diaminobenzenesulphonic Acid** (also known as **Sulfanilic acid**, CID: 8468): ``` Nc1ccc(S(=O)(=O)O)cc1N ``` |
| Beilstein Reference | Beilstein 1678982 |
| ChEBI | CHEBI:28258 |
| ChEMBL | CHEMBL2151327 |
| ChemSpider | 20716 |
| DrugBank | DB14283 |
| ECHA InfoCard | 03f9c804-e4e3-4fcd-8b74-249b5fafe4da |
| EC Number | 226-430-9 |
| Gmelin Reference | 145399 |
| KEGG | C06386 |
| MeSH | D02.241.223.115.132.050 |
| PubChem CID | 10665 |
| RTECS number | SP8750000 |
| UNII | TJ95791T73 |
| UN number | UN2583 |
| CompTox Dashboard (EPA) | DTXSID7020481 |
| Properties | |
| Chemical formula | C6H8N2O3S |
| Molar mass | 216.23 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.58 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.18 |
| Vapor pressure | 1.81E-10 mmHg at 25°C |
| Acidity (pKa) | 1.18 |
| Basicity (pKb) | 7.86 |
| Magnetic susceptibility (χ) | -48.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.683 |
| Dipole moment | 4.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −130.3 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1319 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | D06BA06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H317 |
| Precautionary statements | Precautionary statements: P261, P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | > 210 °C |
| Lethal dose or concentration | Lethal dose or concentration: LD50 oral (rat): 770 mg/kg |
| LD50 (median dose) | LD50 oral rat 1470 mg/kg |
| NIOSH | SE0825000 |
| REL (Recommended) | REL (Recommended): 0.1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Sulfanilic acid Metanilic acid 4-Aminobenzenesulfonic acid 1,3-Phenylenediamine Benzenesulfonic acid |