Out of all the sulfonic acid derivatives of xylene, 2,4-xylene sulfonic acid stands out for its interesting history. Chemists first dug into this molecule’s potential back when coal tar and aromatic organic chemistry dominated laboratory benches in Europe. With industrialization humming along, processes like sulfonation—rooted in old-school dye and detergent production—brought new versatility to xylene compounds. By the early twentieth century, efforts to make functional, water-soluble versions of hydrophobic aromatics led chemists to focus on mono-and di-sulfonated xylenes. As demand for specialty chemicals spread into electronics, pharmaceuticals, and agrochemicals, the value of such tailored molecules soared, bringing 2,4-xylene sulfonic acid right onto the factory floor. Companies have refined both the route of synthesis and the end applications, but those changes rest on decades of laboratory experimentation that gave shape to process safety and scalability.
2,4-Xylene sulfonic acid comes from toluene’s close cousin, xylene, after deliberate chemical transformation. The final product serves as an acidic functional intermediate, playing a key role in the production of dyes, surfactants, and certain specialty polymers. The acid enables the introduction of sulfonic groups into aromatic systems, delivering the right blend of reactivity and stability needed in complex chemical syntheses. From plasticizers to brightening agents, the acid’s reach runs deep, offering manufacturers a springboard into advanced material platforms or tailored molecule production. Those years spent optimizing reactivity and handleability mean modern users rarely run into processing delays.
The solid appears off-white to beige, with hygroscopic qualities. Water loves to stick to it, and it dissolves quickly, pushing its ionic sulfonic group into solution. The acid packs a punch with low pH in dilute solution. It melts between 90–120 degrees Celsius, with decomposition happening before real boiling. Though it anchors to the xylene backbone, adding that sulfonic group transforms volatility and odor into something more manageable for industrial handling. Its high polarity and affinity for water set it apart from its xylene roots. Stability under normal conditions makes it suitable for industrial use without demands for cold storage or elaborate protection from air. Corrosivity remains, so manufacturers work with plastic-lined drums and resistant piping where bulk transfer happens.
Producers sort lots based on purity, moisture content, and free acid levels. The high-purity benchmark sets a minimum of 98%, while water content remains below a percentage point. Color affects customer acceptance; so, some factories add decolorization steps to hit paler shades. Labels cover hazard symbols, handling precautions, batch number, and manufacturing date. Some even flag key impurities, helping downstream users control process quality. Hazard statements and transport markings march lockstep with global regulations, keeping safety and traceability at the forefront. Each bag or drum displays the recognized chemical name alongside any major product code or synonym, cutting confusion during inventory checks or customs processing.
Chemists charge aromatic hydrocarbons with sulfonic acid or sulfur trioxide. Making 2,4-xylene sulfonic acid often starts with mixed xylene and sulfonates the aromatic ring using concentrated sulfuric acid at moderate temperatures. Temperature control proves crucial—cold enough to reduce unwanted isomers, hot enough for complete conversion. Depending on industrial goals, auxiliaries steer the reaction toward the favored 2,4-isomer over others like 2,5- or 3,4-. After reaction, neutralization and isolation steps remove unreacted xylene and by-product acids. To finish, crystallization or spray-drying may help achieve the right particle form for each user. Spent acid recycling marks a common sustainability measure in larger plants.
Adding a sulfonic acid group to the xylene ring means 2,4-xylene sulfonic acid reacts as both an acid and an aromatic. The most common follow-up transformations exploit its ability to undergo sulfonamide or sulfonate ester formation. Chemists also use it for diazotization, creating coupling partners for azo dyes. Under basic conditions, the acid group turns into its sodium or potassium salt, which dissolves easily and proves more user-friendly for downstream processes. Further substitution or condensation with amines and alcohols opens paths into multiple chemical families. The acid group stands up to moderate oxidation and reduction, but strong conditions can strip it off, returning to the aromatic skeleton.
Every chemical catalog worth its salt lists 2,4-xylene sulfonic acid under various guises: 2,4-dimethylbenzenesulfonic acid, 2,4-xylenesulfonic acid, and system names like 2,4-dimethylarenesulfonic acid. Sodium or potassium salts may crop up as sodium 2,4-xylene sulfonate, while some regions run with trade names or abbreviations for invoice convenience. Accurate naming cuts risk of mix-ups in warehousing, regulatory paperwork, or supply chains crossing language borders.
Industrial operators look out for two dangers: corrosivity and dust inhalation. Sulfonic acids burn on skin and eyes, demanding old-school safety gear—goggles, gloves, full-coverage clothing. Inhaled dust irritates airways, so dust suppression and local exhaust pull their weight. Safety data sheets spell out incompatibility with strong bases and oxidizing agents; accidental mixing can start fires or burst containers. Spill procedures call for absorbent barriers and neutralizing lime or sodium bicarbonate. Emergency showers remain within easy reach of process stations. Waste and effluent streams need treatment or neutralization before discharge — missed compliance brings stiff penalties. Regular worker training and chemical hygiene audits reduce incident rates and unplanned downtime.
The rough edges of the acid’s molecule find value across industries. Textile dye makers rely on 2,4-xylene sulfonic acid for coloration steps; its strong acid group helps anchor pigments to fibers under fast fashion timelines. Detergent and surfactant manufacturers like its performance as a building block, supporting water solubility and foam characteristics. Resin producers find it handy as a hardener or linker for specialty polymers that face high heat or chemicals. Chemical synthesis shops purchase the acid for coupling reactions, transformations to sulfonamides, and further derivatization. Tanners, paper makers, and oilfield service companies all carve out their own use-cases, choosing this intermediate for its reliability and process familiarity.
Recent years show a flurry of patents and studies focused on tweaking sulfonic acid derivatives for green chemistry. Interest grows in solvent-free and lower-emission synthesis, since older routes stir up hazardous vapors and acidic wastewater. Researchers test new sulfonating agents and solid acid catalysts to improve selectivity and process safety. On the application side, novel uses in electronics—everything from conductive polymers to batteries—spark global curiosity. Small pilot plants take center stage for scaling up innovative production ideas, merging continuous flow chemistry with old-school batch processing for more flexibility. Chemists keep pushing the limits by functionalizing the molecule for specific reactivity, unlocking new possibilities in catalysis and biocompatible materials.
Across toxicity screens, 2,4-xylene sulfonic acid lands somewhere between household acids and industrial solvents. Animal studies place acute oral and inhalation toxicity in the moderate range, prompting regulators to call for handling controls but stop short of full-on hazard labeling seen with more infamous sulfonates. Skin and eye contact produce strong irritation; repeated unprotected exposure can lead to dermatitis or upper respiratory issues. Workers receive routine monitoring of exposure levels in manufacturing zones, with medical surveillance for symptoms tied to accidental inhalation or ingestion. Environmental impact gets serious attention—aquatic ecosystems react poorly to both the acid and high-salt waste streams formed from its neutralization, driving demand for controlled release, neutralization, or end-of-pipe treatment.
The next chapter for 2,4-xylene sulfonic acid shakes out at the intersection of green chemistry and new electronics. Companies invest in process intensification—making more product with less waste, less energy, and lower emissions. Interest grows in using bio-derived aromatics for greener feedstocks, nudging the acid from fossil-based supply. Applied research in advanced membranes, energy storage, and pharmaceutical intermediates hints at rising demand for tightly specified, ultra-pure versions. Regulatory trends and customer audits continue to shape both the ways products are made and the documentation needed to sell them. As process engineers and synthetic chemists roll out inventive methods for making and using the acid, 2,4-xylene sulfonic acid should maintain its seat among the flexible, go-to intermediates of fine and performance chemical manufacturing.
Anyone who has spent time in a plant or a lab knows that every big product relies on a host of smaller, lesser-known ingredients. 2,4-Xylene sulfonic acid is a perfect example. It pops up in all sorts of chemical processes, quietly making things work better behind the scenes. Walk around any industrial zone that deals with resins, dyes, or detergents, and you’re bound to find it doing some heavy lifting.
More often than not, folks focus on the color at the end of the process, but there’s a reason dyes don’t fade or lose their punch during use. 2,4-Xylene sulfonic acid acts as a sulfonating agent, helping add the right groups to those dye molecules. With this in place, the end product bonds better to fabrics. What you see are clothes or plastics that keep their color through tough washes or long stretches under the sun. The stability comes from the groundwork these agents provide.
Anyone who has mixed up a batch of industrial or household cleaner probably knows the challenge of making things blend together. 2,4-Xylene sulfonic acid plays a hand in turning raw materials into sulfonate salts. These salts help detergents cut through grease, lift stains, and rinse away smoothly. It makes for products that don’t just look good in the bottle, but actually break down dirt and oil reliably in every day use—whether it’s a restaurant kitchen or your laundry room.
People don’t often think about what keeps their water clean. Behind the tap, the water treatment industry depends on complex chemistry. 2,4-Xylene sulfonic acid helps produce chemicals that keep the water clear of buildup and fouling inside pipes. Municipal water systems and cooling towers benefit because the acid supports the production of dispersants. These keep minerals and organic gunk from sticking together or clogging up large infrastructure. In tough climates where scaling is a headache, that’s money saved and less downtime for repairs.
In the world of oil and gas, getting the most out of each barrel matters. Refineries use 2,4-Xylene sulfonic acid to prepare additives that boost the properties of fuels and lubricants. Some of these help oil flow better and reduce deposit buildup inside engines. Out in the field, enhanced oil recovery relies on surfactants based on sulfonic acids. They help loosen stubborn oil from underground rock, pushing yields higher without always resorting to new drilling. In a time of fluctuating energy prices, every bit recovered counts.
Like with many chemicals, responsible handling matters just as much as performance. Factories and companies use strict storage protocols and training to protect workers. Regulations and best practices help keep emissions and waste under control. Many suppliers now look for cleaner production techniques and ways to recycle byproducts. It’s not just good for the environment—it keeps costs down and ensures brands live up to today’s higher standards.
The chemical industry keeps evolving, pulling older compounds like 2,4-Xylene sulfonic acid into new applications. Research teams scan for ways it can help in specialty polymers, advanced textiles, and even pharmaceutical production. As more industries push for performance and sustainability, versatile chemicals like this one stick around by proving their worth where it counts.
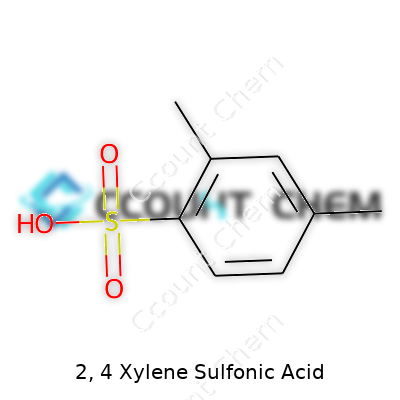
Taking a close look at 2,4-xylene sulfonic acid involves a bit of chemistry, but the logic is nothing out of reach. Start by picturing xylene, an everyday petrochemical ingredient in the paint and adhesive world. Xylene comes in three forms—ortho, meta, and para—depending on where the two methyl groups sit on the benzene ring. For 2,4-xylene, methyl groups stick to carbons two and four on that ring. Add a sulfonic acid group (–SO3H) onto this structure, and that’s where the magic of 2,4-xylene sulfonic acid comes alive.
The resulting compound features that aromatic benzene ring, two methyls on carbons two and four, and a sulfonic acid group often attached to carbon one. Its chemical formula is C8H9SO3H or, more precisely, C8H10O3S when written as a molecular formula. Don’t let all those letters and numbers intimidate you—each part shows how many atoms of carbon, hydrogen, oxygen, and sulfur connect together to shape this useful molecule.
2,4-xylene sulfonic acid does not stay in the background. Chemical plants, pharmaceutical labs, and even municipal water systems depend on this compound. I’ve seen it show up in dye chemistry, helping produce colors that last and resist fading—no small deal for a textile manufacturer. On a personal note, tackling stubborn stains can lead back, indirectly, to surfactants connected to xylene sulfonic acids. Without compounds like this, some detergents wouldn't work as hard in breaking down grease.
Beyond cleaning, this acid plays a part in polymer manufacturing and certain custom resin systems. Chemical engineers value its predictable reactivity and how it blends with other chemicals to make processes smoother. Reliable performance under demanding pressure is something companies never take for granted.
You don’t have to trust the label blindly. Labs confirm the formula using techniques like nuclear magnetic resonance (NMR) and infrared spectroscopy (IR). These tools allow chemists to see exactly where each group attaches on the ring. Running a mass spectrometry check gives them the molecule's weight—if results show 186 g/mol, you know you’re dealing with C8H10O3S. As someone who worked with quality control in a paint facility, I relied on these numbers to clear or reject shipments. The margin for error drops to nearly zero with these technologies.
Handling acids—especially ones related to xylene—demands respect. Protecting workers means proper gloves, eyewear, and fume hoods every time the bottle gets opened. You’ll also find detailed safety data sheets (SDS) with each drum. In my experience, ignoring those guidelines isn’t worth the risk, as even minor exposure can cause lasting irritation or worse.
Environmental regulations around sulfonic acids have grown tighter for good reason. Wastewater from these compounds can harm aquatic life if not treated. Many companies install specialized filtration and neutralization stepping in to keep rivers and lakes clean. Teaching new staff why disposal matters—beyond rules and fines—helps build a culture of responsibility. Strong training shows results in better health and cleaner communities.
Chemists keep working on greener ways to make sulfonic acids, reducing waste and risks. Catalysts that lower energy needs or make reactions more selective bring real improvements. In the field, safer packaging and real-time monitoring help avoid accidents. I’ve watched research teams push for processes that give more product with less byproduct—good for profit and the planet.
People who work in labs, factories, and water treatment plants might run across 2,4-Xylene Sulfonic Acid. Most folks outside those circles don’t need to think about it, but this compound shows up in dyes, detergents, and other chemicals that make modern industry tick. Given its widespread use, plenty of questions start swirling about safety and toxicity.
2,4-Xylene Sulfonic Acid doesn’t hide its sharp smell or bite. Direct contact stings the skin and eyes, leaving burns or irritation. If someone inhales a strong dose, it can hurt the nose and throat and trigger coughing. Cases of accidental exposure don’t pop up as often in public news but in my years talking to chemists and plant operators, skin contact makes up the bulk of problems. A splash, a forgotten glove, or a spill can turn a routine shift into a visit to the medical station.
Researchers haven’t called this acid a carcinogen, but that doesn’t mean exposure is harmless. Most regulatory agencies classify it as hazardous for transport and storage. That includes the U.S. Occupational Safety and Health Administration and the European Chemicals Agency. Both highlight risks for corrosion and irritation.
At home, you’re not likely to encounter this acid straight out of a barrel. Still, in communities near chemical plants or river outflows, environmental release counts as a real issue worth paying attention to. Spillages or poor waste handling can hurt aquatic life, since many sulfonic acids alter water pH and may poison fish or insects.
Some words get tossed around in discussions about chemicals—hazard, toxicity, risk—but they don’t always mean the same thing. From my own time in chemical safety training, hazard describes the built-in danger. By comparison, toxicity looks at how much harm it does inside the body after exposure. 2,4-Xylene Sulfonic Acid stings on contact and can cause local injury, but toxic effects once inside the body appear to be less dramatic than say, heavy metals or solvents like benzene.
Yet, nobody wants to gamble. Long-term effects haven’t been fully studied, so chronic exposure could mean problems that don’t show up right away. Sensitive groups—like people with asthma or skin conditions—should be especially careful around it.
Clear instructions and proper gear turn most hazardous chemicals into manageable tools. In my visits to plants, I’ve seen the difference between good training and careless shortcuts. Goggles, gloves, and sturdy aprons help, but regular drills matter just as much. Quick action in case of spills or splashes often means the difference between an incident and a crisis.
At the community level, strict storage standards keep barrels contained, and monitoring programs watch for spills or releases into the environment. Problems arise when companies cut corners or skip routine inspections—usually in facilities struggling with budget or lacking oversight. Government penalties and public scrutiny push companies to clean up their act, but real progress follows from a culture of safety built into daily routines.
Switching out hazardous acids isn’t always simple. Cheaper or safer options may not always hit the mark for every process, especially in dye or detergent manufacturing. Still, plenty of innovation is underway. Greener alternatives and better recycling technology promise to make a dent. Until then, attention to detail—from the storage closet to the wastewater discharge—keeps both workers and neighborhoods protected.
Real safety doesn’t come from one-off fixes. It comes from constant vigilance and a willingness to respect the hazards right in front of us.
Few chemicals test a facility’s sense of responsibility quite like 2,4-Xylene Sulfonic Acid. This strong acid won’t forgive half-hearted handling. I remember walking into a job years ago and the first thing my supervisor drilled into us was respect for the tools and ingredients around us—especially the ones you don’t want on your skin or in the air you breathe. The pungent nature of this material reminds you to stay alert.
I’ve seen warehouses cut corners thinking nothing will go wrong. Shortcuts don’t pay off. For 2,4-Xylene Sulfonic Acid, choose a cool, dry corner with steady temperature—direct sunlight and moisture ramp up risk. Acid can react with water and certain metals, so metal drums raise eyebrows. Go for high-grade plastic or glass. My experience has taught me that plastic containers, clearly labeled and always upright, cut out a lot of future headaches.
Labels must stay sharp and legible. A faded warning breeds distraction and error. Experienced staff recognize another rule born from experience: keep acids far away from bases or reactive agents. You don’t want cross-contamination turning an accident into a disaster. Suit storerooms with drainage to catch spills before they become dangerous puddles. Ventilation matters too. Noxious fumes have snuck up on many a worker; proper airflow pulls them away before reaching breathing level.
Gloves, goggles, and long sleeves aren’t decorations—they’re the line between a routine shift and a painful ER trip. Splash risk sticks around no matter how careful you get, and chemical burns heal slow. Chemical-resistant gloves and full-face shields buy you the margin of error every worker deserves. A quality apron goes a long way. I’ve seen co-workers wince for days after skipping personal protective equipment during just ‘a quick pour’.
Respirators matter in tight or poorly ventilated spots. The acid’s vapor, inhaled in even small quantities, can inflame the lungs. Regular mask checks keep everyone honest because that gear only works if it’s actually being worn.
Factories sometimes roll out a training day, then forget about it until an inspection appears on the calendar. Ongoing training cements good habits. Employees need real scenarios, not just bullet points from a chemical data sheet. I’ve seen fire drills and spill response practice prevent confusion when the real thing hits. Standard operating procedures posted nearby become lifelines if an accident happens.
Spills happen in the best-run shops. Keeping spill kits stocked with neutralizers and absorbents reduces panic if the worst comes calling. Quick cleanup keeps risks manageable. In my time, nobody ever regretted over-preparing for a chemical accident—every regret follows ignoring the preparation. Waste disposal depends on local laws, but one rule travels everywhere: never pour this acid down the drain. Environmental risk outlasts any short-lived convenience.
Too many operations see chemical safety as checking off a list. The reality feels different in a place where staff watch out for each other and take time to do things right. Pride in a safe shop builds over time, and the payoff shows in productivity and peace of mind. Respect for materials like 2,4-Xylene Sulfonic Acid means healthy workers, clean audits, and none of those late-night calls nobody wants to answer.
Most people who don’t spend their lives around chemical supply catalogs might think acid is simply acid—a jug with a percentage, good to go. That picture loses its neat edges once you start talking about commercial 2,4-xylene sulfonic acid. This compound, known by folks handling detergents, dyes, and specialty chemicals, doesn’t pop up in the same conversation as kitchen vinegar. From experience in an analytical lab, small differences in purity mean big swings for both processing and safety.
Manufacturers typically ship 2,4-xylene sulfonic acid as a concentrated aqueous solution. Most drum labels list purity between 90% and 93% by weight, and that isn’t accidental. Go any higher, and it starts to collect in a gummy mess that’s tough to dose and harder to store. Drop it too low, the efficiency in pigment processing or surfactant formulation starts taking a hit, batch after batch. The rest of the drum usually holds water and a slice of sodium sulfate, with only minor traces from the xylene fraction itself or leftover raw stuff.
Commercial supply isn’t the same thing as pharmaceutical. Producers juggle volume, consistency, and price. Keeping that purity window tight isn’t just for the scientists wearing lab coats—it’s for folks downstream who need predictable results. A deadline-driven plant manager cares about the way low-purity acid might gum up filtration or force a do-over, burning through time and budget.
I remember wasted nights in the lab trying to pin down whether a failing batch of dye finished poorly because of sloppy handling or the wrong grade of sulfonic acid. Surprises often came from impurities—a tiny bit more unreacted xylene or extra water shifted everything. Some users demand certificate of analysis sheets before even opening the drum, which keeps the process honest. It’s not just the printed figure, but also by-products and trace ions that show companies know what landed in their barrel.
Quality control, as dull as it sounds, is where headaches get cut off early. Many buyers ask for batch samples, or rely on reputable distributors who ship verified lots, not lowball gray-market mixtures. Failing to do so, people risk process hiccups, off-spec outputs, and, in some sectors, safety warnings. Acid this strong eats through open skin and corrodes spill trays, making mislabeling a risk no one wants.
Some solutions sit close to home. Suppliers who collaborate with buyers—taking feedback, adjusting purity specs for each application—tend to stick around. Automated titration and better monitoring tech keep concentrations on target, which leads to fewer surprises. Open records and full transparency from the supplier side, matched by user-side inspections, leave less room for costly mistakes and skepticism.
High-purity 2,4-xylene sulfonic acid isn’t just marketing; it’s a promise that less goes wasted. Factories cut overtime, researchers avoid repeat experiments, and cleanup crews see fewer acid burns. It’s not a glamorous chemical, but it proves how much riding on a simple number can steer a whole industry’s profit and reputation.
| Names | |
| Preferred IUPAC name | 2,4-dimethylbenzenesulfonic acid |
| Other names |
2,4-Xylenesulfonic acid 4-Methyl-2-toluenesulfonic acid 2,4-Dimethylbenzenesulfonic acid Benzenesulfonic acid, 2,4-dimethyl- 2,4-Xylylsulfonic acid |
| Pronunciation | /tuː fɔːr zaɪˈliːn sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 1300-72-7 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2,4-Xylene Sulfonic Acid**: ``` CC1=CC(S(=O)(=O)O)=CC=C1C ``` |
| Beilstein Reference | 695734 |
| ChEBI | CHEBI:132741 |
| ChEMBL | CHEMBL135637 |
| ChemSpider | 161385 |
| DrugBank | DB13183 |
| ECHA InfoCard | 100.010.423 |
| EC Number | 253-185-9 |
| Gmelin Reference | 1774 |
| KEGG | C07475 |
| MeSH | D015874 |
| PubChem CID | 91702 |
| RTECS number | ZE9625000 |
| UNII | 588LU9I50G |
| UN number | UN2586 |
| CompTox Dashboard (EPA) | DTXSID4078946 |
| Properties | |
| Chemical formula | C8H10O3S |
| Molar mass | 186.22 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.26 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -0.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.5 |
| Basicity (pKb) | 4.41 |
| Magnetic susceptibility (χ) | -60×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.576 |
| Viscosity | 20-40 cP |
| Dipole moment | 3.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 273.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -492.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3914.7 kJ/mol |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P264, P280, P301+P330+P331, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | 230°C |
| Lethal dose or concentration | LD50 (oral, rat): 720 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1450 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m3 |
| Related compounds | |
| Related compounds |
o-Xylene p-Xylene Benzene sulfonic acid Toluene sulfonic acid Xylenol 2,4-Xylene sulfonyl chloride |