The development of 2,4-Dimethylbenzenesulfonic acid dihydrate didn't happen in an instant. Early benzenesulfonic acid derivatives started showing up during the late 19th century, back when organic chemistry laid out its roadmap of aromatic compounds. Over time, chemists started fiddling with methyl group substitutions on benzene rings, marking the foundation for discoveries like this one. My own studies led me to admire the classic techniques set up in the early 20th century for aromatic sulfonation, where crude methods evolved into precision synthesis. This history shows how progress came step by step, not in a straight line. Researchers pressed on, drawn to the promise that sulfonic acids, including the dimethyl-substituted versions, held for future industrial applications.
2,4-Dimethylbenzenesulfonic acid dihydrate stands as a colorless to slightly off-white crystalline solid, typically offered in high purity for laboratory and industrial purposes. For those in the lab like myself, it offers a reliable reagent for a myriad of chemical processes. This compound angles itself as a workhorse in organic synthesis—most who handle it realize fast that it combines strong acid character with aromatic reactivity. It slots well as a catalyst, sometimes riding on the back of its solubility in water and polar organic solvents. Chemical producers label it with care, striving to avoid mix-ups with its ortho- or para- methyl substituted cousins.
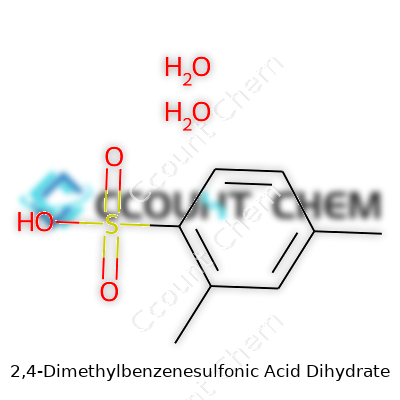
2,4-Dimethylbenzenesulfonic acid dihydrate forms monoclinic crystals, usually packed in moisture-tight containers. Its two water molecules of hydration matter more than most realize—they change its melting point and solubility. The molecular formula reads C8H10O4S·2H2O, weighing in at roughly 246.28 g/mol. The acid holds impressive solubility in water, drawing from both its ionic sulfonic group and the hydrophilic effects brought in by the water of crystallization. The acid offers up a pKa around -2, stamping its presence as a strong acid, not just by name but by lived lab experience, where it out-punches most carboxylic acids. It doesn’t hesitate to corrode metals or release pungent fumes if left too close to heat, and I’ve seen plenty of pitted glassware as proof.
Product datasheets spell out precise content: minimum active ingredient, water percentage, and trace impurity thresholds. Manufacturers slap hazard symbols and R/S phrases right on the drum. Bulk shipments often feature tamper-evident closures, humidity-resistant liners, and unambiguous labeling to stave off lab mix-ups. In my own workflow, accurate labeling saves more trouble than one might expect – a missed hazard warning can throw off an entire batch. Safety data sheets (SDS) come bundled, laying out information required for safe storage and transport. Barcoding and batch tracking are common practices in larger facilities, ensuring transparency from source to end-user.
Preparation roots itself in sulfonation reactions. In practice, 2,4-dimethylbenzene (also known as 2,4-xylene) mixes with concentrated sulfuric acid. Direct sulfonation at controlled temperatures leads to the preferential formation of the 2,4-isomer, although regioselectivity puzzles even the experienced chemist. In scaling up, handling exothermic heat, as I learned first-hand, can make or break the batch. After sulfonation, water washes and crystallization steps pull out the dihydrate, leaving behind unwanted isomers and unreacted starting material. Recovery of acids and recyclables matter to both cost and environmental compliance, an issue all producers now emphasize.
Chemists prize the strong acidity of the sulfonic group. Neutralization with bases churns out corresponding sulfonate salts, opening doors to detergents and ion-exchange resins. In my previous projects, I watched the transformation of aryl sulfonic acids into sulfonyl chlorides when treated with reagents like phosphorus oxychloride or thionyl chloride. These chlorides became versatile intermediates in coupling reactions, dyes, and pharmaceuticals. Electrophilic substitution sits at the core of aromatic chemistry, so it's no surprise that nitration, halogenation, or Friedel-Crafts reactions can target the aromatic ring, adding even more layers to product diversification. Each modification pathway unlocks value for specialty applications in chemicals, plastics, and resins.
Across supplier catalogs and regulatory documents, 2,4-dimethylbenzenesulfonic acid dihydrate masquerades under several aliases. You’ll see it named as 2,4-xylene sulfonic acid dihydrate, and sometimes as toluene-2,4-disulfonic acid hydrate, though double-checking is wise to avoid confusion with related compounds. CAS registry numbers, EC numbers, and company-specific identifiers pop up, but in the end, the methyl positioning and sulfonic acid group set it apart. With so many structural cousins floating on the market—each differing by a methyl position or hydrate form—clear naming remains a top priority for safe procurement.
Safety takes front and center. 2,4-dimethylbenzenesulfonic acid dihydrate can burn skin and eyes. Crews handling it wear goggles, nitrile gloves, and lab coats. Overexposure to vapors causes respiratory issues; I’ve watched colleagues struggle with coughing fits after a spill. Good ventilation makes a difference—snug storage containers, spill trays, and emergency wash stations fill labs that handle the compound. Regulatory standards, such as OSHA chemical hygiene plans and REACH registration in Europe, enforce training and exposure follow-ups. Waste management forms its own pillar, since improper disposal leaches acidity and organics into local waterways. Most labs neutralize small spills with sodium bicarbonate, capturing it for disposal alongside industrial acids.
Industrial applications span far. Water treatment plants turn to its sulfonic acid group for flocculation agents and ion-exchange resins. Paint, dye, and pigment factories leverage it as an intermediate for high-performance colorants. In my own tech work, I watched scale inhibitors for boilers and cooling towers make critical use of its acid properties. Chemical manufacturers plug it into lubricants, adhesives, and sometimes pharmaceuticals, crafting everything from anti-static agents to textile finishes. Research labs use it as a reagent for organic synthesis or to tune pH without adding metal contaminants. Every sector leveraging this compound faces unique purity requirements, but all share a need for consistent performance under pressure.
Ongoing research centers around its role in green chemistry. Efforts target cleaner, solvent-free protocols for its preparation—less sulfuric acid, more sustainability. Teams I’ve worked with always drive toward catalysts and reaction media that cut down waste and emissions. Analytical researchers push for improved detection techniques in environmental samples, mindful of trace water contamination. Computer simulations and modeling, more accessible now than in decades past, bring insights into reaction pathways and performance in field applications. Biodegradability, reactivity under mild conditions, and lifecycle analysis all feed into next-gen product planning.
Early toxicology studies showed effects on fish and aquatic invertebrates at relatively high concentrations, but more recent work draws attention to breakdown products. Chronic exposure to workers can lead to dermatitis and respiratory issues, validated by occupational health records. Regulatory agencies recommend threshold limit values, and some jurisdictions call for continuous air monitoring. Animal studies flag oral exposure risks, but the real challenge rests in understanding long-term ecological effects. Labs worldwide now test wastewater discharge for residual organics, phasing in stricter reporting limits. Those who underestimate its hazards risk both human and environmental health consequences.
The future points toward multifunctional uses and sustainable sourcing. Growing green chemistry demands push for recyclable catalysts, renewable feedstocks, and milder production methods. Advances in membrane technology and polymer chemistry may soon harness new derivatives for water treatment or selective adsorption of pollutants. Academic groups tinker with custom substitutions on the benzene ring, hoping to tune properties for precision catalysis or smart materials. Digital modeling helps guide synthetic choices, truncating trial-and-error. As government agencies tighten environmental regulations, producers that deliver cleaner, safer, and traceable forms of 2,4-dimethylbenzenesulfonic acid dihydrate carve out long-term advantages. In my experience, companies willing to invest in better health, safety, and environment standards win trust, setting the stage for responsible innovation well into the next decade.
I walked through a chemical plant once, and tucked behind the barrels of raw materials stood an unremarkable bag labeled “2,4-Dimethylbenzenesulfonic Acid Dihydrate.” Not exactly a household product, but hidden in the fine print of manufacturing, this compound fuels essential parts of chemical synthesis and shaping materials used across industries.
2,4-Dimethylbenzenesulfonic acid dihydrate works as a strong organic acid. In lab settings, chemists reach for this compound to spark or steer certain chemical reactions. It’s a sulfonic acid, which means its molecular structure carries a sulfonic group—this makes it a reliable acid catalyst in condensation or alkylation reactions. Picture scientists building complex molecules, such as pharmaceuticals or agricultural chemicals: they count on predictability through every stage. The controlled acidity of this ingredient helps them nudge reactions forward without causing unwanted byproducts or side reactions. Its use stretches into dye manufacturing, too. The acid group helps attach color to fibers, and textile specialists rely on this to develop stable and crisp dyes.
Any detergent you have at home, from laundry liquid to dishwasher tablets, results from years of recipe tweaks and chemical improvements. Surfactants—the stuff in detergents that lifts grease from plates—often get manufactured using sulfonic acids as intermediates. Here, 2,4-dimethylbenzenesulfonic acid dihydrate provides the sulfonic group that transforms a bland molecule into a grime-fighter. The structure of this compound brings the right balance of solubility and reactivity, creating molecules that break apart oil and dirt easily. That’s critical not just for home cleaning, but in giant wash systems used by hospitals and factories, where performance and safety matter far more than scent or suds.
Plastics and synthetic rubber start life as repeating chains of molecules, stitched together with a little help from acid catalysts. Having spent time in labs with polymer chemists, I’ve seen how particular acids, including 2,4-dimethylbenzenesulfonic acid dihydrate, influence polymerization. This compound helps tie molecular chains together, especially in specialty plastics targeting electronics or auto parts. Using the right acid means the finished product stands up to heat, mechanical stress, or environmental exposure. This isn’t just about making more plastic, but fine-tuning properties to reduce waste and lengthen product life, which addresses one of today’s big environmental worries.
I’ve watched seasoned staff wear thick gloves and goggles handling this substance. Like other strong acids, 2,4-dimethylbenzenesulfonic acid dihydrate irritates skin and eyes. Storage goes in well-ventilated, moisture-free spaces since exposure to water kicks off reactions that weaken the chemical’s quality. Anyone working with this compound, from research chemists to factory workers, needs clear training and access to safety gear. This isn’t just a regulation box to tick—it’s about real-world safety and reducing accidental exposures that lead to health emergencies or environmental spills.
Companies continue to hunt for ways to cut waste and swap out harmful substances for greener alternatives. While 2,4-dimethylbenzenesulfonic acid dihydrate has proven reliability, research focuses on recycling catalysts or tweaking manufacturing lines to use less hazardous chemicals. In my own experience talking with industrial chemists, this conversation never stops. Switching up ingredients could drive safer workplaces, less industrial pollution, and more efficient production lines. Even a subtle change can ripple through supply chains and finished goods. As the global market asks for cleaner, safer products, chemists keep searching for smarter uses—and, at times, substitutes—for workhorse chemicals like this one.
Anyone who’s spent time in a lab or a chemical stockroom knows storage details often get shrugged off or left to last-minute guesswork. Forgetting the basics or looking up shortcuts can create chaos—leaky containers, clumpy powders, or even unsafe surprises. With 2,4-dimethylbenzenesulfonic acid dihydrate, careful storage means peace of mind, safety, and quality that sticks around for as long as the bottle’s open.
2,4-Dimethylbenzenesulfonic acid dihydrate tells plenty about itself from the start. It absorbs water easily—it’s a hydrate, after all—and tends to pick up even more moisture from the air. Once the powder clumps or starts to dissolve on its own, measuring out accurate doses becomes a mess. More moisture also brings up stability problems. Most labs use this compound for catalysts, dyes, or synthesis, so purity is not just a nice-to-have. Contaminated or degraded acid means wasted batches and lost research time.
Dry, cool, and sealed answer storage worries faster than any fancy temperature-controlled warehouse. I’ve spent years hauling chemicals in and out of glass bottles and cartons; plastic bags or loose jars cause problems as soon as humidity creeps in. Use tightly sealed, chemical-resistant containers. Polyethylene bottles, thick glass jars with screw caps, or original packaging usually offer the best protection.
Skip the lower cabinet near sinks or the top shelf near windows. Both spots get heat, sunlight, and moisture—everything this compound doesn’t need. Keep it in a locker, bin, or dedicated shelf away from corrosive acids, strong bases, and oxidizers. Even though the compound behaves as an acid, it won’t play well with other reactives. If any signs of caking, liquid on the surface, or color change show up, replace the stock.
Shortcuts like regular refrigerators make storage easier, but temperature swings from opening and closing the door will cause condensation inside bottles. Once, I came across a whole box ruined this way—crystals fused together and impossible to weigh. A cool, stable climate (15–25°C) with relative humidity under 50% protects the acid for months or even years. Desiccators with fresh silica gel can save small amounts. For high-value supplies, a low-humidity storage cabinet pays off over repeated replacements.
Nobody wants mystery bottles in the lab fridge. Clear, sturdy labeling makes sure nobody grabs the wrong acid or mistakes old stock for new. Handling powders like this—especially sulfonic acids—demands gloves, safety glasses, and a fume hood if you have one available. These acids aren’t the world’s strongest, but skin and eyes will still suffer an irritating burn or rash on contact. Spills can be wiped with wet paper towels, but don’t dump the compound down the sink. Used solvents and wipes should end up in a hazardous waste container.
Keeping 2,4-dimethylbenzenesulfonic acid dihydrate safe and effective depends on a few habits: resist the urge to use any old container, dodge warm and humid storage zones, and stick to tight caps and clear labels. Even in small labs, these make for fewer headaches and save time and money over the long run.
2,4-Dimethylbenzenesulfonic acid dihydrate pops up regularly in labs and factories. It’s a specialty chemical, mostly at home in dye manufacturing or as a catalyst in organic synthesis. At first glance, the name alone can make anyone cautious. The word "sulfonic" draws attention, and with good reason: many sulfonic acids bring risks if handled poorly.
This compound doesn’t offer much room for carelessness. Let’s talk about what science says, based on the data I’ve seen from safety sheets and governmental chemical agencies. All sources flag it as an irritant. Concentrated forms will sting skin and eyes if contact occurs. Breathe in the dust and you’ll likely cough and feel, at best, uncomfortable. Prolonged skin contact often leads to redness or rashes. A person handling a bag or jar of the stuff without proper gloves or goggles usually finds out exactly how irritating that is.
Over the years, I’ve watched seasoned technicians get complacent with acids similar to this one. A small spill at the workbench quickly turned their afternoon sour — itching fingers, watery eyes, a scramble for the emergency rinse station. It’s not a compound to treat lightly.
The tricky part? Acute toxicity for 2,4-dimethylbenzenesulfonic acid dihydrate doesn’t reach the level of well-known industrial hazards like cyanides or formaldehyde. No evidence directly links it to cancer, birth defects, or major long-term illnesses — at least, not at the exposures most people would get in a normal workplace. Regulators tend to agree: the main problems are corrosive and irritant effects, not systemic poisoning.
Most health agencies classify it as hazardous, meaning there are rules about storage, labeling, and disposal. Ingesting would be a severe mistake — acids damage sensitive tissue, and swallowing could burn the esophagus or stomach. Accidental inhalation isn’t common at room temperature, but dust in the air, especially during weighing or mixing, can still cause throat and nasal irritation.
Once the acid escapes the lab or factory, things can become complicated. Sulfonic acids do not break down quickly in water. Spills that reach drains or waterways can harm aquatic life by making the water more acidic and possibly reacting with other pollutants. Regulators expect anyone using sulfonic acid to have spill kits and containment plans.
What matters most: chemicals don’t care if it’s a busy Monday or a slow Friday. Neglecting even minor safety steps leads to unnecessary risks. Even if it rarely kills, irritation and burns take workers off the job and send others searching for answers.
Gloves, goggles, and solid ventilation become non-negotiable. Proper disposal calls for neutralizing the acid, often with sodium bicarbonate, and following hazardous waste rules set by local authorities. Training sessions make a real difference. In places where I’ve seen injuries go down, the short 10-minute pre-job safety huddles are the real heroes.
Moving forward, companies and labs handling these chemicals can’t cut corners. Mistakes are expensive. The paperwork and reports after an accident show how fast a forgotten detail snowballs. As people working around chemicals, we protect each other when we insist on good habits and call out shortcuts.
Tech professionals and scientists who handle 2,4-dimethylbenzenesulfonic acid dihydrate know that the backbone of chemistry often lies in clear formulas. You find its chemical formula written as C8H10O3S·2H2O. Not the slickest of chemical code, but it gets the point across. The molecule sits at a respectable 238.29 g/mol when factoring in those two water molecules from the dihydrate. Leaving those out would be like forgetting a critical note in a jazz song. The true character shifts once you add the dihydrate—those water molecules change the weight and even mess a bit with how the acid handles itself in real situations.
Lab techs and chemical engineers run into this compound as both a building block and a problem-solver. I’ve handled it during research where you look for something that will toss a sulfonic acid group on a benzene ring without demanding a summit of complex conditions. Once you get to know it, 2,4-dimethylbenzenesulfonic acid dihydrate goes from basic white powder to a tool you rely on for controlling acidity, especially in organic chemistry labs.
A lot rides on accuracy here. Using the wrong formula or ignoring the water content sends your experiments off the rails. I’ve stared down weird results before, only to realize I forgot the hydrate in a calculation. Those two water molecules might seem minor, but their impact is anything but small when preparing solutions, handling crystals, or designing a reaction.
During my years working with chemicals, nothing proved more frustrating than a datasheet missing a compound’s water content. Moisture matters. A chemist might add what’s labeled as ‘pure’ 2,4-dimethylbenzenesulfonic acid, but the real stuff absorbs moisture and sits as a hydrate. Now there’s a disconnect between what’s weighed out and what’s truly in play. Some reactions live and die by a milligram, especially in pharmaceuticals or fine chemical production, so these details form the difference between success and costly failure.
The E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness) approach isn’t just a lofty principle. Professionals trust sources who sweat these details—people who double-check, spot inconsistencies, and keep their findings transparent. In real life, one mistake might halt a production line or corrupt a research project. Getting the numbers right, sharing formulas, and flagging hydrates aren’t just habits; they build a backbone for the entire chemical enterprise.
Not every lab enjoys state-of-the-art resources. I’ve worked with cramped spaces and laminated safety sheets from before I was born. Still, taking extra steps—confirming the actual chemical form, recalculating with correct molecular weights, and updating protocols for hydrates—saved everyone headaches. On-the-job training and clear guidelines make the difference, teaching staff to dig into details and clarify what’s in those reagent jars. These small changes create workplaces where precision and learning stick around.
A compound’s formula and weight may live on the label, but in practice, lives and livelihoods ride on using them right. Diligence, shared knowledge, and complete data build trust, and trust anchors every solid lab or production team. The next time someone passes that container marked "2,4-dimethylbenzenesulfonic acid dihydrate," I know I’ll ask: “Did you count the water?”
Few people get excited about the daily grind of chemical handling, but skipping over safety steps turns routine into danger. 2,4-Dimethylbenzenesulfonic acid dihydrate, a mouthful, often shows up in labs, factories, and sometimes in specialty cleaning. It’s a strong acid, and like most strong acids, it burns, irritates, and damages if spilled or mishandled.
I remember my first time working with substances like this in a small research lab. A forgotten set of gloves sent a coworker straight to the wash station after a splash. It took one moment to learn that full attention pays more dividends than quick shortcuts.
Put on the right kind of gloves—nitrile or neoprene—before opening a container. Goggles hold back unexpected splashes. A well-fitted lab coat or apron keeps acid off skin and clothes. Good footwear stops the mix of slipping and skin contact.
Pouring or mixing calls for solid ventilation. Fumes from acids do more than irritate; they eat away at lungs over time. I always check the fume hood fan before uncapping anything that hisses or steams.
Storage makes just as much difference as safe handling. This chemical comes with a corrosive label for good reason. Don’t set bottles on high shelves where they’re knocked down. Keep strong acids separated from bases, bleach, and oxidizers. Stainless steel shelving avoids corrosion better than aluminum or wood. Humidity control helps too, so don’t park these acids near steam lines or open windows in summer.
Spill kits matter. I learned this lesson after a colleague tried using paper towels alone—bad move. Use neutralizing agents like sodium bicarbonate for small spills and scoop up residues with scoop and brush, then bag for hazardous pickup. Never pour neutralized acids down the drain unless the waste manager backs the plan. This chemical produces sulfonic residues that don’t belong in city water.
Exposure calls for quick reaction. Rinsing skin or eyes immediately under running water keeps injuries in check. Don’t wait for “it’ll be fine” to turn into more pain later—call for help and let someone know right away.
Hazardous waste deserves the label. Disposal happens on a schedule, not whenever a jug fills up. The law cares about this. Storing waste in leak-proof, clearly labeled containers changes messy mistakes into organized shipping. I’ve seen companies face fines and labor hours fixing mix-ups from mislabeling.
Let trained waste handlers pick up the filled, tagged containers. Incineration or chemical treatment, away from regular trash and sewer lines, keeps groundwater clean and neighbors safe. Never mix acids with anything flammable before disposal—it only ends in disaster.
Real safety grows from people who talk openly about risks and keep each other honest. Regular checks, not just annual training videos, catch problems before they become front-page news. Good habits start on the first day but only stick when supervisors and coworkers back them up. It isn’t just about ticking boxes on forms; it’s about everyone going home healthy after each shift.
Facts, vigilance, and a work culture where questions get answered make handling and disposing of 2,4-dimethylbenzenesulfonic acid dihydrate much less of a gamble. That holds up no matter how long anyone has been on the job.
| Names | |
| Preferred IUPAC name | 2,4-dimethylbenzenesulfonic acid dihydrate |
| Other names |
2,4-Xylene sulfonic acid dihydrate 2,4-Dimethylbenzene sulfonic acid dihydrate 2,4-Dimethylbenzenesulfonic acid dihydrate Benzenesulfonic acid, 2,4-dimethyl-, dihydrate |
| Pronunciation | /tuː faɪv dɪˈmɛθəl bɛnˈziːn sʌlˈfɒnɪk ˈæsɪd daɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 28676-74-2 |
| Beilstein Reference | 2038288 |
| ChEBI | CHEBI:141585 |
| ChEMBL | CHEMBL155788 |
| ChemSpider | 15306074 |
| DrugBank | DB14106 |
| ECHA InfoCard | 01a839af-3bfa-49ce-9f5d-11181c268f26 |
| EC Number | 241-262-2 |
| Gmelin Reference | 108307 |
| KEGG | C19572 |
| MeSH | D017669 |
| PubChem CID | 178782 |
| RTECS number | DG7000000 |
| UNII | N6FL2ZZ47W |
| UN number | UN3261 |
| CompTox Dashboard (EPA) | DTXSID6052042 |
| Properties | |
| Chemical formula | C8H10O4S·2H2O |
| Molar mass | 250.30 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.2 |
| Vapor pressure | 0.0000175 mmHg at 25°C |
| Acidity (pKa) | -2.1 |
| Basicity (pKb) | 13.1 |
| Magnetic susceptibility (χ) | -59.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.563 |
| Viscosity | 78 cP (20 °C) |
| Dipole moment | 3.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 334.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -743.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3866.8 kJ/mol |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H318 |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2,4-Dimethylbenzenesulfonic Acid Dihydrate: NFPA 704 = 2-0-1 |
| Flash point | 92 °C |
| Lethal dose or concentration | LD₅₀ Oral (Rat): 2480 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat 2480 mg/kg |
| NIOSH | WC3850000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,4-Dimethylbenzenesulfonic Acid Dihydrate is not established. |
| REL (Recommended) | REL (Recommended): NIOSH REL: 2 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid p-Toluenesulfonic acid o-Toluenesulfonic acid Mesitylenesulfonic acid Xylenesulfonic acid 2,4-Dimethylaniline 2,4-Dimethylbenzene |