Chemists started tuning molecules like 2-(2-Sulphonatoethyl)Isothiouronium long before anyone worried about digital precision or automation in the lab. This compound’s roots stretch back to the days when textile coloring and dye-fixing called for sharper, faster, and more wash-resistant bonders. Isothiouronium groups interested researchers in the early twentieth century, thanks to their knack for forming tight bonds with negatively charged dyes on fibers. Over time, research peeled back layer after layer—finding utility for these sulfur-containing molecules in everything from dye intermediates to biochemistry. The industrial shift toward safer yet more functional sulf(on)ic acids encouraged the creation of sulfonated isothiouronium derivatives. By the 1980s, factories across Asia and Europe ran this product as a staple in their chemical inventories, not just for textile use but as a fixative and intermediate in medical, photographic, and analytical chemistry applications.
2-(2-Sulphonatoethyl)Isothiouronium often comes up in conversations about specialty chemicals destined for industrial processes that require durability in harsh conditions. This compound is basically an isothiouronium core with a sulfonate tail and a flexible ethyl spacer bridging the two. The sulfonate head attracts water, giving the molecule good solubility—an important feature when working with aqueous systems. Isothiouronium chemistry pulls double duty: it not only reacts with a variety of nucleophiles, it also displays strong cationic behavior in water, which matters in both textile treatments and biochemistry settings. Chemical catalogs sometimes list it alongside other dye fixatives, but its uses travel well beyond just dye houses; medical diagnostics, reagent kits, and surface modification chemistry all have a place for it.
2-(2-Sulphonatoethyl)Isothiouronium usually forms as a white or slightly off-white crystalline powder. It dissolves readily in water, thanks to the strong hydrophilicity of its sulfonate group. The melting point lands above 200°C, consistent with other sulfonic acid derivatives of similar size. Chemists value its stability in acid and neutral solutions. The molecule stands up reasonably well to moderate heat but decomposes on strong heating—producing sulfur dioxide, cyanide-type compounds, and other breakdown products, so operational care becomes important. The odor typically stays mild, nothing like the more notorious thiol relatives.
Producers ship this compound according to international chemical safety standards, with packaging labeled for purity, batch number, synthesis date, and country of origin. Most suppliers aim for purities over 98%. Since the product can absorb moisture from the air, proper packaging involves moisture-barrier bags or drums. The molecular weight stands at 195.25 g/mol. SDS (Safety Data Sheet) documentation cites its water solubility, irritant risk on eye and skin contact, and lack of volatility. Labeling sometimes uses shorthand like “ISOU-ethyl sulfonate” depending on the tradition in a local market. Long names get shortened for use in tracking, storage, and process documentation to avoid confusion.
Synthesis of 2-(2-Sulphonatoethyl)Isothiouronium begins with 2-bromoethanesulfonic acid or its sodium salt as starting material, which reacts with thiourea under controlled heating conditions. The reaction borrows from classic nucleophilic substitution: thiourea attacks the bromoethyl group, threading together the isothiouronium and sulfonate functionalities. After the reaction, chemists wash out excess reagents and unwanted byproducts using water, followed by precipitation and filtration. Quality-minded producers adjust pH, dry the material under vacuum, and screen for purity using both wet chemistry (like titration) and instrumental analysis (like NMR or HPLC). Consistency between batches depends on precise temperature control, stoichiometry, and cleanup.
Once in-hand, 2-(2-Sulphonatoethyl)Isothiouronium offers several reactive outlets. The isothiouronium end reacts with a variety of nucleophiles, forming guanidine derivatives under good yields. The compound also participates in Michael additions and can migrate to different positions on polymer backbones. In textile processing, it latches onto anionic dye groups, essentially locking color to fabric. More adventurous researchers tweak its structure, adding bulkier groups for new cationic surface modifiers or swapping in different aromatic backbones to sharpen its reactivity. Photochemists occasionally graft it onto light-responsive polymers, using the sulfonate as an anchor.
Plenty of catalogs list this chemical under names like “2-(2-sulphoethyl)isothiouronium,” “isothiouronium 2-sulfoethylate,” or just “SEITU.” The diversity in names arises from naming conventions in different regions and end-use markets. CAS registries sometimes call it “ethanesulfonic acid, 2-(isothiouronium)ethyl ester.” The naming helps with regulatory tracking and customs declarations, a constant challenge in global chemistry supply chains. Still, the molecular fingerprint stays consistent—a sulfonic acid group tethered by two carbon atoms to the isothiouronium core.
In real-world plants and labs, workers handling this compound suit up with goggles, gloves, and splash-resistant lab coats. Factories often set local exhaust or fume hoods around discharge points. On contact, the powder irritates eyes and, less often, skin; ingestion is a risk best left untested. Storage standards prevent water ingress, and fire safety guidance emphasizes that although this chemical itself won’t catch fire easily, it can emit toxic gases during decomposition. Emergency protocols typically highlight first-aid rinses and medical evaluation for direct exposure. GHS (Globally Harmonized System) labeling applies—usually with mild hazard icons.
Most conversations about this compound trace back to the textile and paper industries. Mills count on 2-(2-sulphonatoethyl)isothiouronium to fix reactive and direct dyes onto cellulose fibers, reducing dye leaching and keeping washfastness high. Dye fixatives, in my memory of factory visits, essentially keep colored jeans from bleeding out in the first home wash, a real money-saver for brands and a relief for consumers. Beyond textiles, this molecule’s positively charged core gives it a spot in medical research, acting as a cross-linker for certain proteins and enzymes. Some labs use it in microarray technology, where it helps bind biological probes to plastic slides. Electroplating formulas sometimes sneak in sulfonated isothiouronium derivatives to improve metal adhesion or alter surface charge.
Not everything about 2-(2-Sulphonatoethyl)Isothiouronium is routine. Research teams at specialty chemical companies keep looking for ways to wring out more performance—longer shelf life, faster reactivity, or greater compatibility with environmentally friendly dyes. Environmental regulations in the European Union and North America drive a lot of this work, pushing for lower toxicity and better breakdown rates in wastewater. University projects in China and India dive into tweaking the compound for biosensor coatings or as a building block in water-soluble polymers aimed at drug delivery. I recall a handful of startups exploring its use as a linker for nanoparticles, eager to solve adhesion problems on inorganic surfaces. Basic research has focused on tuning its pKa and exploring new coupling strategies for enzymes and peptides.
Toxicologists approach this compound with sensible caution. Animal model studies show low acute oral toxicity, though concentrated exposures cause irritation to the gastrointestinal tract, mucous membranes, and skin. Chronic exposure data remains thin, although breakdown products after combustion—cyanide and sulfur dioxide—raise flags for fire safety and regulatory teams. Environmental testing in the last two decades measures how much of the compound escapes dyehouses and whether it shows up downstream in water treatment plants. Results show that like many sulfonated organics, it persists moderately well but doesn’t bioaccumulate substantially. Regulators call for standard handling protocols, SDS access, and spill plans as a matter of best practice.
Growth for this compound depends on a few obvious trends. Green chemistry goals put pressure on older, more stubborn dye fixatives, so greener alternatives or improved derivatives of 2-(2-Sulphonatoethyl)Isothiouronium will find a bigger market share. Water treatment systems need molecules that both adhere firmly to contaminants and can later break down into innocuous fragments—incentivizing new research on hybrid isothiouronium compounds. Biomedical device industries and specialty plastics continue to demand new surface coating technologies for diagnostics and implants, a spot where this molecule’s adaptability could shine. As global supply chains become more transparent and regulatory standards tighten, manufacturers will lean on data-driven quality control, lifecycle analysis, and the results of deeper toxicology screenings before mainstreaming new applications.
Most people don’t think about what goes into getting a crisp white shirt into their closet, but behind every smooth piece of cotton, there’s often 2-(2-Sulphonatoethyl)Isothiouronium working away. Known in textile factories as an effective antistatic and desizing agent, this compound makes the work easier and safer. Cotton tends to pick up static electricity, especially in large machines. Sparks put workers and products at risk. By adding 2-(2-Sulphonatoethyl)Isothiouronium during the processing, static clings get tamed and safety improves. I’ve visited textile plants where problems with static once caused frustration and lost time, only to see the shift when operators switched to using this chemical.
The compound doesn’t stop with antistatic uses. Dyers and finishers appreciate it for more reasons. Cotton and wool both often resist dyes. This substance helps “fix” the dye so colors look sharp and stay that way through plenty of washes. Garments that keep their color after months in the wardrobe are getting help from chemicals like this one. The textile industry as a whole looks for products that comply with environmental and safety regulations; this compound often finds a spot because manufacturers trust its profile and performance.
Textile manufacturers looking for conductive yarns or fabrics also lean on 2-(2-Sulphonatoethyl)Isothiouronium. By treating fabrics, they can offer antistatic uniforms, protective gear, or materials for electronics—and reduce electronic interference. I remember a project where static shocks would damage sensitive components; switching to antistatic-treated gloves made a difference overnight.
This compound doesn’t just stick to cloth. Factories handling water treatment sometimes use it for its properties as a biocide and as a corrosion inhibitor for metal pipes. Boilers receive this chemical in their feed lines because it stands up to heat and keeps corrosion at bay, reducing downtime from repairs and ensuring water runs without interruption. Fewer shutdowns mean less disruption downstream—important for small towns and big cities alike.
Industrial chemicals bring benefits, but health and environmental safety always remain a top concern. 2-(2-Sulphonatoethyl)Isothiouronium, like many similar agents, shouldn’t get released unchecked into effluent streams. Facilities under-regulate use risk local water pollution and push up cleanup costs for communities nearby. Factories working under ISO 14001 standards and those audited regularly usually keep close watch on discharge, running effluent through treatment systems before sending any byproduct downstream. Solutions often come down to fitting proper filtration and monitoring equipment, plus ongoing staff training to spot leaks or accidental spills quickly.
There’s a real demand for textiles and manufacturing methods that don’t sacrifice the planet for profit. Some companies have started exploring plant-based or enzyme alternatives but haven’t matched this compound’s reliability in all applications. Industry leadership groups keep pressing for more studies, tighter sourcing, and transparent labeling so buyers can check for safety and environmental protection along each step of the supply chain.
Awareness is growing—among factory workers and management alike. The next step lies in investing in research, sharing best practices, and holding everyone in the production chain to high standards. With sharper oversight and open communication, the textile and treatment industries can keep benefits high and risks low.
Anyone with a curious mind and some background in science has wondered how certain chemicals get their unique properties. 2-(2-Sulphonatoethyl)Isothiouronium is a good example of a molecule that shows how chemistry can solve practical problems in daily life. Its structure helps decide where it fits into the big picture of applications, from textile processing to the world of analytical chemistry. Anyone who’s spent time working around lab benches will know compounds like this have a heavier presence than many realize.
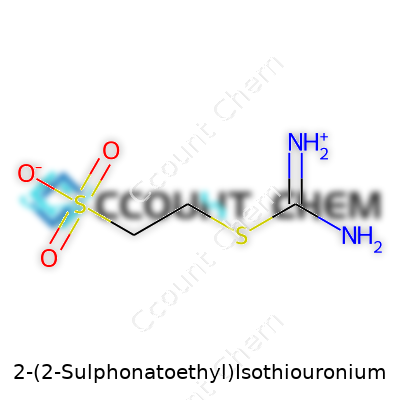
The backbone of this molecule starts with a simple two-carbon chain—an ethyl group. On one end, you’ll find a sulfonate group (SO3-), which gives the compound its water-loving nature. Chemists call this functional group “hydrophilic," but the take-home point is clear: it easily dissolves in water. Sticking a sulfonate group on a carbon skeleton helps the compound move smoothly in aqueous environments, which is useful for reactions or products requiring water solubility. I’ve personally seen how products that wouldn’t dissolve otherwise can become workable after adding sulfonate-containing substances.
The other end of the ethyl chain features the isothiouronium group. Structurally, this portion looks like this: –NH–C(=NH2+)–S–. Here, sulfur links to a positively charged guanidinium-like group. This section makes the molecule capable of binding to negatively charged ions, like chlorides and certain metals. Anyone who’s handled specialty reagents in water purification or dye-fixation can confirm the importance of these positively charged zones: they cling to what needs removing or fixing, creating opportunities for new products or better performance.
Put these pieces together: Ethyl provides the backbone, sulfonate brings water solubility, and the isothiouronium group packs in reactivity. The overall molecule looks like this when drawn out: SO3-–CH2–CH2–S–C(NH2)2+. This arrangement ensures both positive and negative charges exist in different sites of the molecule. Many chemists call these “zwitterions." Having both charges matters for compatibility. It keeps the compound stable across a range of pH environments. This is no small thing if you’ve ever had to troubleshoot a finicky dye bath or wrestle with unpredictable water hardness in an industrial setting.
In textile factories, 2-(2-Sulphonatoethyl)Isothiouronium gets used to lock dyes onto fabrics, making colors last longer and resist fading. Its dual charges and water solubility let it move through fabric and find the right binding spots. Even on a lab scale, those who analyze trace metals in water have relied on molecules like this to selectively grab impurities.
People sometimes overlook risks connected to specialty chemicals. Safe storage and careful handling can’t be an afterthought, especially with compounds sporting sulfur groups, which occasionally break down and release odors or toxic byproducts. Relying on personal experience, keeping good records of what’s on the shelf and wearing the right protective gear reduces risk in the lab or on the shop floor.
To improve outcomes, training staff about properties and reactivity helps. Selecting the right storage materials—preferably glass or compatible plastics—cuts down on unwanted reactions. Reviewing waste disposal protocols for sulfonate and isothiouronium compounds prevents contamination and environmental issues. Investing time in preparation saves headaches and money down the line, whether managing research spaces or overseeing manufacturing lines. Responsible handling paired with a strong grasp of the chemical’s structure opens more doors for safe, productive use in industry and research.
In labs and factories, people run into chemicals most folks have never heard of. 2-(2-Sulphonatoethyl)Isothiouronium pops up in specialty industries—electroplating, textile processing, sometimes in research. It’s not a household name, but that doesn’t mean it is harmless. Working near or with this material, safety isn’t a luxury; it’s a non-negotiable routine.
Wearing gloves and goggles aren’t just for show. When exposed without protection, this compound irritates skin, eyes, and the lungs. Touch it, and the skin reacts—redness, itching, or even burns with enough contact time. Breathe in the dust or fumes long enough, and the respiratory system deals with coughing, sneezing, or sore throat. The problem isn't only about personal discomfort. Extended exposure boosts the risk of developing allergies, making reactions stronger with each encounter. The body remembers.
Animal studies evaluating sulfur-based isothiouronium compounds reveal moderate toxicity levels. Oral exposure causes symptoms like nausea, headache, and gastrointestinal discomfort. Lab rats develop liver and kidney changes at high doses. There’s no credible evidence pinning it as a cancer risk in people, but no medical expert ever recommends regular, unprotected exposure to a chemical with these effects. Worker case studies track problems tied to skin and respiratory irritation, especially where factories skip proper air circulation and personal protection.
After use, waste containing 2-(2-Sulphonatoethyl)Isothiouronium ends up in water or soil, which means local ecosystems pay the price. The compound doesn’t disappear; water organisms become casualties of its presence. Fish exposed in contaminated streams show signs of stress, slower growth, and lower survival rates. The aquatic impact is documented in research done near textile manufacturing hubs. Wastewater treatment helps, but only if plants actually install and maintain effective systems. Without proper disposal, factory runoff adds to the load, putting community health on the line.
Real solutions happen on the ground. Chemical suppliers must give full safety data, including first-aid instructions. Workplaces need forced-air ventilation, gloves, face shields, and regular health checks for exposed staff. There’s no shortcut—training matters. In my time training new staff at an electroplating shop, nobody skipped the basics: wash stations, hazard labeling, and spill kits stood ready by every workstation. Regular audit checks made sure corners weren’t cut. It might sound strict, but accidents drop when awareness goes up.
Engineers have developed new treatment filters specific for sulfur-based compounds. These filters help catch and neutralize toxic waste before it hits waterways. Laws in Europe and North America push industries to keep emissions well below strict thresholds. Enforcement can lag but workers and concerned citizens have eyes on the issue, sometimes pushing regulators when industry falls short.
Whether on a shop floor or overseeing environmental compliance, it pays to take chemical hazards seriously. Protective gear, proper disposal, updated safety training—these are the steps that keep people and the planet safer. With substances like 2-(2-Sulphonatoethyl)Isothiouronium, cutting corners isn’t worth the risk.
Anyone who spends time working with specialty chemicals comes to appreciate one thing: details matter. With 2-(2-Sulphonatoethyl)Isothiouronium, skipping over storage considerations sets you up for expensive headaches. This compound brings its own quirks—a sulphonic acid group and an isothiouronium moiety. These features give it water solubility, but also leave it open to moisture absorption and hydrolysis if ignored on the shelf.
Humidity can swing a solid like this from a dry powder to a sticky mess. My old workplace left an unsealed bag out “just for an hour.” By the end of the shift, that sample looked clumpy and had to be thrown away. Even if the container looks tight, the air left inside can pull in just enough water to cause caking and possibly slow breakdown of the compound. Anhydrous conditions in a dry box—especially in humid climates—actually save headaches, not just “add work.” Desiccators fitted with silica gel or other standard drying agents do a solid job, but only if checked routinely and recharged as needed.
Plastic bottles look convenient, but some additives and softeners aren’t fully compatible with reactive sulfur chemicals. In my own benchwork, I’ve seen glass bottles keep products purer, even after a year. Amber glass wins bonus points by shielding from light. Screw tops lined with PTFE create a tough barrier for leaks and chemical vapors. Avoid tin or iron lids, because isothiouronium groups—like other guanidinium-based reagents—can pick up trace metals, turning once-clear powders discolored and leading to variable results in downstream reactions.
A cool, consistent space gives 2-(2-Sulphonatoethyl)Isothiouronium a long shelf life. There’s no need to jam it in a -20°C freezer, though. Common wisdom puts it between 15‒25°C. I’ve seen colleagues throw it in a fridge “just to be safe.” Not only does this take up valuable lab real estate, but it risks condensation every time the jar is opened. A spot in a temperature-controlled cabinet, out of direct sunlight and away from heaters or vents, works better. If the working space heats up (think attic labs in summer), taking a temperature log can flag risky fluctuations before they cause problems.
Vague, handwritten labels have tripped up labs more than once. A permanent marker and clear info—product name, concentration (if in solution), lot number, and date received—cuts confusion when your research group outgrows its storage bins. Recapping thoroughly after scooping out material keeps oxidation and moisture at bay, and reduces incidents of accidental exposure. Trust me, nothing ruins an afternoon like discovering powder at the bottom of your backpack.
Loading up every new shelf without thinking leads to surprise fires and ruined reagents. 2-(2-Sulphonatoethyl)Isothiouronium reacts badly with strong oxidizers—peroxides, permanganate, and nitric acid. Storing bottles separately from such chemicals reduces hazard, making audits and fire risk assessments easier. Using secondary containment trays builds in another layer of spill protection, and doubles as an early warning if leaks ever show up.
Simple rules—keep it dry, give it its own clean bottle, store cool, label well, and keep it away from the hotshots of oxidation chemistry. The peace of mind that comes from never having to toss a spoiled batch or look for missing data is worth the few extra minutes up front.
Chemists talk a lot about molecular weights, but for those who don’t spend their days in a lab, that phrase might seem a bit abstract. It means adding up the atomic weights of every element in a compound’s chemical formula. With 2-(2-Sulphonatoethyl)Isothiouronium, the formula comes out as C3H9N2O3S2. It’s got three carbons, nine hydrogens, two nitrogens, three oxygens, and two sulfurs.
To figure out the total, you grab the atomic weights: carbon (12.01), hydrogen (1.01), nitrogen (14.01), oxygen (16.00), sulfur (32.07). Tally those with their counts.
Pile those up: 36.03 + 9.09 + 28.02 + 48.00 + 64.14 lands you at 185.28 g/mol. That number anchors all sorts of decisions, from quality control in manufacturing to dosing in experiments.
During my years around university chemistry benches, I saw how a single wrong calculation could lead to wasted batches or confusing results. If someone misread a label or skipped a decimal, an experiment could fail or produce data that didn’t make sense. Chemicals like 2-(2-Sulphonatoethyl)Isothiouronium often show up as specialty reagents in dyeing or textile treatments. In these applications, molecular weight tells you precisely how much to weigh out to get the right number of molecules reacting in your process.
I remember working with a student who swapped two digits on a molecular weight sheet. The whole batch came out off-color and uneven. That’s not just an inconvenience; it means lost time, wasted resources, and sometimes strict safety checks to verify residue amounts. Mistakes like this also create ripple effects throughout research, as papers relying on shaky calculations propagate flawed data.
By keeping an eye on the molecular weight, labs keep chemicals in the right range for processes like purification or blending. Any deviation affects more than just the final look of a product. It can impact workplace safety, environmental release, and regulation as well. In fact, major chemical incidents often start with calculation errors or poor labeling—simple mistakes that connect back to paying close attention to foundational numbers.
Trade groups and regulatory bodies recommend regular audits and in-house cross-checks for compound identities and molecular weight calculations. One smart habit involves a “double-check day” where another chemist reviews all critical data. The pharmaceutical industry takes this to heart, using barcoding and digital records to root out human error.
It helps to digitize reference tables, use software to verify manual math, and cultivate a workplace culture where double-checking is standard. Younger scientists benefit from seasoned researchers showing them pitfalls through real-life stories. Creating hands-on learning moments beats memorizing tables in isolation every time.
Getting molecular weights right—like 185.28 for 2-(2-Sulphonatoethyl)Isothiouronium—runs deeper than textbook exercises. The number ripples through everything, from cost estimates to the ethics of safe reagent handling. Reliable answers start from details, and molecular weight stands up as one of the most basic, most important details in the field.
| Names | |
| Preferred IUPAC name | 2-(sulfonatoethylcarbamothioyl)azan-1-ium |
| Other names |
2-(2-Sulphonatoethyl)isothiouronium inner salt 2-(2-Sulfoethyl)isothiouronium |
| Pronunciation | /tuː tuː sʌlˈfeɪtəʊ ˈiːθɪl aɪsəʊˌθaɪjʊˈroʊniəm/ |
| Identifiers | |
| CAS Number | 12167-46-1 |
| Beilstein Reference | 626840 |
| ChEBI | CHEBI:38746 |
| ChEMBL | CHEMBL1230498 |
| ChemSpider | 2924018 |
| DrugBank | DB04261 |
| ECHA InfoCard | 03b8efc5-20ff-4571-8d5a-ade2e2b14eae |
| EC Number | 212-661-7 |
| Gmelin Reference | 82156 |
| KEGG | C05524 |
| MeSH | D021237 |
| PubChem CID | 21559656 |
| RTECS number | WN8300000 |
| UNII | 545MI06E78 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID6047277 |
| Properties | |
| Chemical formula | C3H9N2O3S2 |
| Molar mass | 242.30 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.439 g/cm3 |
| Solubility in water | Very soluble in water |
| log P | -4.3 |
| Vapor pressure | Vapor pressure: <0.01 hPa (20 °C) |
| Acidity (pKa) | -3.6 |
| Basicity (pKb) | pKb = 3.15 |
| Magnetic susceptibility (χ) | -22.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.475 |
| Dipole moment | 5.5 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 208.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -566.35 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -709.6 kJ/mol |
| Pharmacology | |
| ATC code | V03AB38 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0-X |
| Lethal dose or concentration | LD50 Oral Rat 710 mg/kg |
| LD50 (median dose) | LD50 (median dose): 825 mg/kg (oral, rat) |
| NIOSH | SW7525000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-(2-Sulphonatoethyl)Isothiouronium is not specifically established by OSHA. |
| REL (Recommended) | 1% |
| Related compounds | |
| Related compounds |
Thiourea Isothiourea Sulfanilic acid Ethanesulfonic acid 2-Mercaptoethanol |