Chemistry follows a winding road, full of detours and sudden breakthroughs. The origins of 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt trace back to the practical needs of synthetic and analytical chemists in the 20th century. Research groups, especially those focused on water solubility and coordination chemistry, kept pushing sulfonic acid derivatives into new territory. Over time, the drive for water-stable reagents led to the systematic study of bisethanesulfonic acids and their salts. It wasn’t a single “aha” moment but the steady accumulation of lab notebooks, failed batches, and patient iteration that pushed this compound from curiosity to staple. Keeping track of small details—temperature at each step, the influence of batch impurities—turned out to make a real difference in how this salt matured from lab talk to production reality.
Anyone handling specialty reagents comes to appreciate the details: 2,2’-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt stands out for its niche role as a buffering agent, a ligand in analytical assays, and a stabilizer in biochemistry protocols. People in the lab know this salt by its punchy specificity—it delivers high solubility and predictable reactivity in water-based environments. The product usually appears as a fine, free-flowing powder, cream to off-white in color. It rarely clumps or cakes when stored properly. So, lab workers can count on consistent handling from bottle to bench, which matters on those grinding mornings when reliability can make or break a procedure.
Few things separate a lab-grade reagent from a headache like knowing exactly what you’re working with. This disodium salt presents high solubility in water—which comes from the two sulfonic acid groups readily ionizing in solution. Molecular weight circles around 285 g/mol, and the melting point edges higher than 280°C, stable for most practical lab conditions. Hydroscopic tendencies often pop up, so containers with tight seals become a simple necessity for maintaining quality. Chemists note its strong hydrophilicity, shaped by the sulfonate and hydroxyimino groups, which also set up reactive possibilities. Solutions made with this salt remain clear even after standing, which speaks to its purity and minimal tendency for precipitation.
Commercial suppliers stamp out detailed technical sheets: assay (by titration or NMR) generally guarantees purity above 98%. Guidelines typically specify storage in cool, dry places, with labeling that lays out the molecular formula (C4H6N2Na2O7S2), batch number, and expiration date. Safety icons—oxidizer, irritant—crop up to nudge proper handling, since direct skin or eye contact invites irritation. Packaging standards use HDPE drums or glass jars, keeping the compound shielded from ambient moisture during transport or long-term storage. In regulated labs, tracking lot numbers on the label helps in tracing back performance issues or cross-contamination risks.
Getting this salt into pure form means chasing efficiency through multi-step synthesis. Starting with ethylenesulfonic acid, labs introduce oxime groups via nitrosation followed by neutralization. The disodium salt forms by slow, controlled addition of sodium hydroxide. Controlling the pH at each reaction step keeps side reactions limited, and careful crystallization, often under reduced temperature, locks in product quality. Filtration, wash steps, and drying finalize the process. Chemists expect some trial and error—reaction yield often bumps against the limits set by reagent-grade purity and water quality.
The hydroxyimino moiety opens doors to selective transformations—complexation with transition metals, mild reductions, or further sulfonation come to mind. Collaboration between inorganic and organic chemists has led to tailored modifications, such as alkylation or coupling with peptide backbones, to broaden biological compatibility. The compound resists mild oxidants and bases, staying reliably inert in most buffer systems, but stronger acids or reducing agents can break it down. Over time, research groups develop custom modifications to fine-tune binding constants, supporting projects in catalysis or protein purification. Each tweak must be balanced against stability and cost—too much modification, and storage headaches quickly appear.
Depending on who you ask, lab catalogs list this salt as BIS-TRIS, Bistrisoxime, or just “Disodium bis(2-sulfoethyl)oxime.” CAS numbers, usually 123239-01-2 or similar, often provide the clearest reference through regulatory and procurement channels. Brand-specific names sometimes pop up, but most researchers stick to IUPAC names or standard abbreviations in peer communication. This green-labeled ambiguity works fine in conversation but can trip up less-experienced users, especially during ordering or cross-checking protocols.
Lab safety depends on the small choices made at every step. Working with this compound, direct inhalation or skin exposure brings mild irritation, and eye exposure burns more seriously. Standard PPE—gloves, goggles, dust mask—becomes an easy habit. Spills mix readily with water, so neutralizing and diluting with plenty of rinse solves most bench messes. Waste needs careful collection, since sulfonic acids and their salts don’t always mix well with heavy metal waste streams. Most regulatory rules file it as low-to-moderate hazard, so regular chemical hygiene plans cover storage and waste disposal. MSDS sheets sit close at hand, and handling guidelines stress not eating, drinking, or smoking near reagent prep stations.
The salt’s greatest strength? Water solubility and chemical stability. Labs put it to work in biological buffers, analytical titrations, and ligand design. Its chelating capacity helps separate metal ions in trace analysis, cutting down on noise in spectrophotometry or chromatography assays. Molecular biologists lean on its buffering power to hold pH steady, whether prepping proteins or performing enzyme reactions. Pharma researchers experiment with its stabilizing effects, trying to preserve drug or enzyme activity across batches. Water treatment engineers sometimes try the compound in pilot projects seeking selective ion capture. Real-world adoption depends less on grand theory and more on whether a supplier can keep up with batch-to-batch consistency.
Academic labs remain curious about the ways oxime and sulfonate groups interact in solution. New studies poke at the binding affinities for transition metals, looking for clues that might unlock new catalysts for pollution cleanup or green chemistry. Pharmaceutical research tests the salt’s impact on enzyme stability and protein crystallization, banking on the idea that a well-chosen buffer sets up more reproducible results. Efforts turn to making the salt from sustainable feedstocks, since petrochemical-derived sulfonic acids run into both cost and public perception problems. On the academic side, peer-reviewed articles dig into NMR spectra, stability in mixed solvents, and compatibility with biological systems.
Most animal and cell line studies show low acute toxicity for pure 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt. But researchers watch for chronic effects, metabolic pathways, and break-down products. The salt clears quickly from most biological tissues, but incomplete clearance in high-dose scenarios still raises concerns. Eye and mucous membrane irritation limits its use in clinical or direct-contact medical devices. Long-term safety studies, especially those looking at environmental fate in wastewater streams, still find gaps in current data. Environmental scientists push for better characterization, especially as sulfonic acid derivatives become more common in industrial and consumer products.
Looking at the long game, 2,2’-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt stands well-poised for new applications. As biotech, water treatment, and pharmaceutical manufacturing ramp up, the demand for water-soluble, non-toxic, and stable reagents will only grow. Automation and AI-driven process design in chemistry may unlock hidden potential in its use, pushing for custom-tailored molecules based on this simple but sturdy scaffold. More sustainable production methods and full life-cycle safety studies will likely define its place in green chemistry efforts. Research communities keep driving towards greater understanding, not only in how it works but in what makes it a better fit for environments outside the classic chemistry lab.
Most folks haven’t heard of 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt, but it pops up in biochemistry labs more often than you’d think. I remember my workbench days, poring over protocols, where this mouthful of a chemical made frequent appearances. Known by its nickname HOBES, this compound steps into the spotlight when scientists want reliable, nontoxic buffering for experiments. HOBES shines because it holds pH steady, keeping all those tiny but crucial reactions humming along without throwing off the balance.
Plenty of buffers crowd the shelves. Yet, some break down under heat, mess with sensors, or just introduce problems nobody asked for. HOBES brings real advantages to the table. Its low UV absorbance means it doesn’t mess with spectrophotometry readings—a vital part of measuring proteins, enzymes, and DNA in research. Turn on that UV light, and HOBES stays quietly out of the way, delivering accurate results. During my own graduate studies, chasing faint protein signals, this helped cut out noise in my data.
The sodium salt form dissolves fast in water and stays stable—even as temperatures swing. This matters for labs that need to keep results steady from day to day or from one instrument to another. Reliable chemistry supports reproducible science, which ultimately bolsters trust in published findings. Leading journals ask for raw data and repeatability, so using dependable reagents like HOBES becomes even more important under tough peer review.
Enzyme experiments can go haywire if buffers break down or interact with reactants. Certain common buffers, such as Tris or HEPES, might interfere with metal ions or affect the actual chemistry researchers try to study. HOBES keeps mostly out of these battles. Scientists working with sensitive metal-based enzyme systems appreciate the way this buffer won’t grab at calcium, magnesium, or zinc in test tubes.
Biosensor developers favor HOBES for another reason. It resists forming crystals, won’t clog microfluidic channels, and comes clean off glassware—no sticky residue, no weekend lab cleanups. Someone slogging through long days of data collection, hopping between assays and calibration checks, finds smooth-running buffers help support progress and morale in the lab.
No chemical solves every problem. HOBES isn’t always perfect for every protocol. Its synthesis requires careful handling to ensure safety and avoid impurities. Some suppliers cut corners, and trace contaminants can ruin an experiment before it starts. This raises the broader issue of quality control in chemical sourcing. Labs already squeezed by tight budgets often purchase cheaper reagents and gamble with results. I’ve seen grant money evaporate because of bad batches, and students chasing ghosts in their data for weeks. Reliable sourcing networks and open sharing of supplier reviews can curb those setbacks.
Putting more thought into the chemistry behind every experiment pays off in faster breakthroughs and sturdier science. Scientists share best practices through forums, papers, and even social media. HOBES might play a small part on the surface, but its dependable buffering ensures the groundwork for discovery stays strong. The world benefits when tiny details like buffer chemistry support, not sabotage, big ideas. By sharing our experiences and being honest about pitfalls, the scientific community keeps making better choices, for our workbenches and for the results people count on.
Deep in the corners of a laboratory shelf, chemicals like 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt often fade into the background, overshadowed by the busier bottles of everyday reagents. Yet, as someone who spent years making sense of confusing test results and strange reaction behavior, I’ve learned the hard way that how we store specialty salts can turn routine work upside-down.
This compound, widely valued for its role as a buffer and reducing agent, comes as a white crystalline solid, which gives many researchers a false sense of security. Leaving it at room temperature, next to the acids and bases, looks fine on paper, but changes start to creep in. Manufacturers and chemical safety guides recommend keeping it sealed tightly, away from humidity, and stored in a cool, dry environment. A basic rule— shoot for temperatures between 2–8°C. I found simple refrigeration beats the slow headaches that come from decomposition caused by a warm, moisture-rich shelf. At standard room temperature, nobody’s doing long-term storage any favors, especially during summer months or if the central cooling goes out for a day.
Moisture does more than cake up powders and stain labels. For compounds with sulfonic acid groups, like this salt, high humidity invites clumps and can even shift the actual concentration of solutions made later. A room with dehumidification, a working desiccator, or even a dry, airtight container protects against this. Once, a forgotten desiccant packet saved an entire lot of buffer components—simple fixes beat hours of recalibrating experiments.
While this salt holds up against occasional light and air, repeated exposure can nudge degradation, especially for open containers. To keep things straightforward, reaching for amber bottles or dark drawers keeps UV out. Oxygen sneaks in past loose lids, so containers require firm closure every time. From my bench work, failing to seal the container led to unexpected product breakdown, which always takes longer to unravel than just screwing the top back on each time.
Beyond the basic science, proper storage cuts risks in the workplace. Even stable salts can trigger skin irritation or accidental inhalation if spilled from poorly capped bottles, especially when humidity turns powder into paste. Storing these chemicals at eye-level, not on the top shelf or under the fume hood, makes grabbing them safer and faster. That adds up, especially over months in a teaching or production lab.
Regular inventory checks prevent surprises. I found that labeling containers with opening dates and storage locations cleared up confusion about which batch suffered after a day of high heat. Training new staff and students on these simple storage steps keeps good habits alive. And updating safety sheets to reflect real-life storage spots avoids the classic “misplaced in the flammable cabinet” moment.
The importance of proper storage for 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt traces back to every reliable experiment, every accurate measurement, and every safe workplace. Clear refrigeration, dry containers, darkness, and regular checks reduce waste and frustration. A few minutes of care gives days of reliable results—something every scientist, technician, or educator wants from the start.
You find a chemical like 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt mentioned in some research papers or lab supply catalogs, and it can spark a lot of questions. Safety at work or in the lab doesn’t happen by accident—it starts with asking the right questions. “Is this stuff safe?” sounds simple. With less common chemicals, the answers rarely feel simple or easy to find.
I remember standing at a lab bench as an undergrad, staring at a label I could barely pronounce. It’s easy to think that if you haven’t heard of a chemical before, it’s probably not a big threat. Yet, that’s rarely true. Dig into available safety data for 2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt, and the readings are thin. There’s no big stack of case studies, nor does it often show up on major chemical hazard alert lists. That doesn’t mean it’s as innocent as table salt.
Most widely used safety references—like the GHS classification system or databases from regulatory bodies—have scant information on this compound. This limited data usually signals two things: little use outside specialized fields and a lack of major publicized incidents. But limited use often means limited testing, not a guarantee of safety. In my career, I’ve seen compounds with “no data available” labels later shown to be skin or respiratory irritants, sometimes even more. Sometimes, missing data is more warning than reassurance.
Let’s look at its structure. The presence of sulfonic acid groups and hydroxyimino functional groups often brings up concerns about water solubility and reactivity. Water-soluble chemicals have an easier time getting into the skin and can move through water supplies. That alone tells me this compound doesn’t just disappear harmlessly once down the drain. Handling procedures for chemicals like this typically call for gloves, goggles, and good ventilation—even if there’s nothing in the bottle’s documentation about its hazards.
Many related chemicals can be irritants. Some sulfonic acid derivatives provoke eye or skin irritation. Inhalation risks grow during processes like weighing out fine powders or mixing. Open wounds or sensitive skin can turn a minor exposure into a memorable one. Until proven otherwise, I treat every unfamiliar chemical as though it can cause trouble.
Maybe the biggest safety issue isn’t the chemical itself—it’s assuming safety just because there’s little formal documentation. Over the years, I’ve seen too many people regret skipping the extra precautions. General good practices like a working fume hood, tight seals, and not eating or drinking anywhere near handling spots go a long way.
Getting more thorough data is tough. The best shot for the average lab worker is consulting the supplier’s SDS (Safety Data Sheet), but even these sometimes have lots of question marks and blanks in sections that matter. Larger or forward-thinking suppliers sometimes fund further testing, but that only helps if everyone actually takes the time to read those sheets instead of relying on word of mouth or habits.
I avoid touching any unfamiliar chemical directly, keep my protective gear close, and stick to procedures set by trusted sources. I ask questions when I see new bottles or jars in the workspace and pay attention to substitute products, since sometimes a “new and improved” chemical comes with unstudied risks.
In the end, respecting uncertainty keeps more people safe than assuming innocence. Until comprehensive toxicology reports come in—and let’s be honest, they rarely do for lesser-known lab salts—I figure on the side of glove-up, gear-up, and stay alert.
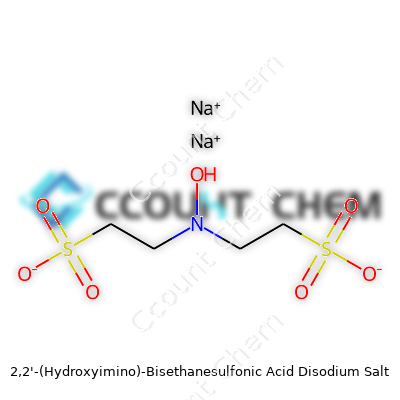
2,2'-(Hydroxyimino)-Bisethanesulfonic Acid Disodium Salt has the chemical formula C4H8N2Na2O7S2. The name looks like a mouthful, but the actual structure tells a story about why researchers care about this compound. Many in the lab simply refer to it by its common name, HOBES. Each molecule packs two ethanesulfonic acid groups, an oxime bridge, and two sodium counterions. This isn’t random design—it’s a tool chemists use for very specific reactions. The molecular weight lands at 324.22 g/mol. This number matters any time someone plans stoichiometry for complex syntheses or scales up a reaction. I remember on more than one occasion, mixing up final molarity calculations just because a sodium salt was overlooked. It’s a mistake that throws off entire batches and wastes both time and resources. Having that figure right in front of you goes a long way.
Accuracy steers chemistry. A missed atom or swapped number wipes out thousands of dollars in time or materials. The four carbon atoms, eight hydrogens, two nitrogens, seven oxygens, two sodiums, and two sulfurs all must balance. Working in the lab, everyone counts on precise molecular weights for weighing reagents. If you grab the wrong salt form of a compound, results shift, sometimes in invisible ways—like yielding unexpected impurities or shifting product distribution. This compound’s sodium salt delivers water solubility—you can’t overlook that detail if you want reactions to stay in solution.
HOBES salts, with their solid sulfonate anchors and oxime chemistry, show up in many biochemical protocols. The strong acid character from the sulfonic acid improves compatibility with water-based systems. Researchers sometimes favor these buffers when working at neutral to slightly alkaline pH. Traditional choices like phosphate buffers get in the way during metal catalysis or protein assays. In our own protein labeling routines, swapping in HOBES allowed us to keep metal ions stable and maintain consistent buffer pH. It’s a reminder that not all buffer salts work the same, and finding the right fit speeds up troubleshooting.
Even outside typical protein conjugation, sulfonic acid salts like this help keep analyte stability high during advanced spectrometric detection or chromatography. Injecting unstable molecules leads to poor readings and reproducibility. The industry increasingly moves toward more robust and reliable analytical chemistry by opting for better-defined, high-purity salts—HOBES being a strong contender.
Precise reporting of weight and formula remains non-negotiable. Forgetting to account for sodium or failing to correct for its presence can derail research and delay development timelines. Laboratories benefit from standardized reference materials for reagents, especially with salts involved in biochemical assays or regulatory studies. Firms stand to gain from better batch QA systems—by logging forms and weights directly into digital workflow tools, fewer mix-ups happen at the bench.
With chemical supply chains getting more global, purity certificates and reliable molecular information build trust in products and help sustain innovation. Catching mistakes at this level—rather than downstream during data collection—saves money, time, and patience.
2,2'-(Hydroxyimino)-bisethanesulfonic acid disodium salt shows up in labs for a range of biochemical research and testing. You can spot it in reagent bottles or powder containers, usually off-white, quietly waiting for someone to weigh it out. In my early days working at a biomedical lab, the supervisor drilled the importance of respecting even less-common chemicals. Too many safety lectures that washed over us at first, until someone mixed the wrong buffer and sent everyone scrambling for the eyewash station. That memory sticks.
Treat this compound with the same seriousness you’d give harsher acids or bases. Use gloves with strong chemical resistance; cheap nitrile often stands up, but I've watched co-workers double glove just in case. A tight-fitting lab coat, closed shoes, and safety goggles turn into your non-negotiables. If the chemical powder dusts up even a little, it lands on hands or cuffs, waiting to ride home with you. Many labs wisely require working near a chemical fume hood for anything more than microgram amounts. Not every workplace boasts a perfect setup, but nobody wants respiratory or skin exposure shaping their career—or their health.
Store the solid always in a cool, dry spot, away from sunlight or anything humid. Most modern labs use desiccators or sealed lockers for this reason. Not only does moisture change the weight and consistency, it can turn a straightforward experiment into a guessing game. I’ve witnessed the leftovers from one mis-sealed bottle—it turned into a sticky mess, calling for a tense afternoon clean-up. Label your container with both the chemical name and date opened, then double-bag it if you share a communal space. Lock cabinets help keep unfamiliar hands away.
Messy spills rarely play out the way textbook scenarios do. If you knock over even a small vial, grab the spill kit without hesitation. Wearing fresh gloves stops the spread. Scoop the powder up with a dedicated spatula and dispose of gloves right afterwards. Wipe the spot down with a damp towel, using as little solvent as possible. One old skill worth passing down: always work outwards from the furthest edge of a spill back toward the middle, so the powder doesn’t get smeared wider. If you think any splashed out of immediate sight, make a note in the lab log or daily report—coworkers trust each other more when everything is put in plain sight.
Waste handling laws get stricter every year, and for good reason. This acid salt will never belong in a drain or trash can. Most campus labs collect unused powder or contaminated gloves in a labeled, sealable container, then send it for hazardous waste pickup. Municipal waste services don’t know what to do with these chemicals; letting professionals handle them reduces groundwater and landfill contamination risks. One of my mentors taught me to always “over-label”—add CAS number, date, and all related hazard warnings to the waste log. Most chemical safety officers appreciate this, and it builds a safety culture everyone depends on. Diluting or neutralizing on-site sounds tempting until you factor in byproducts and downstream effects that nobody can guarantee are safe.
Every lab benefits from routine review of handling and disposal protocols. Set times for retraining, share stories of near-misses, and stay in touch with your institution’s environmental health officer. Once a team shares the expectation that nobody shortcuts disposal steps, the bar rises for everyone. I’ve seen student-run labs transform after a close call, carrying better habits into every new project. With chemicals like 2,2'-(hydroxyimino)-bisethanesulfonic acid disodium salt, the real power comes from becoming stewards, not just users.

| Names | |
| Preferred IUPAC name | Disodium 2,2'-[(hydroxyimino)imino]bis(ethane-1-sulfonate) |
| Other names |
N,N-Bis(2-sulfoethyl)nitroxylamine disodium salt BESNA BES-Oxime disodium salt |
| Pronunciation | /ˌhaɪdrɒkˈsɪmɪnoʊ baɪsˈeθeɪnˌsʌlˈfɒnɪk ˈæsɪd daɪˈsoʊdiəm sɔlt/ |
| Identifiers | |
| CAS Number | 59774-06-0 |
| Beilstein Reference | 87392 |
| ChEBI | CHEBI:137420 |
| ChEMBL | CHEMBL1209428 |
| ChemSpider | 22102311 |
| DrugBank | DB04061 |
| ECHA InfoCard | 03a0c7a7-5945-464f-9473-cf8aa5e5e4cd |
| EC Number | 243-867-2 |
| Gmelin Reference | **Gmelin Reference:** 172299 |
| KEGG | C05775 |
| MeSH | D02.886.590.700.200.500 |
| PubChem CID | 9809093 |
| RTECS number | NJ6825000 |
| UNII | E3M85D75XA |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID2036795 |
| Properties | |
| Chemical formula | C4H6N2Na2O7S2 |
| Molar mass | 311.18 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.348 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.2 |
| Vapor pressure | < 0.01 mmHg (20 °C) |
| Acidity (pKa) | 1.83 |
| Basicity (pKb) | 6.26 |
| Magnetic susceptibility (χ) | −44×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 30 - 70 cP (25°C, 1% in H2O) |
| Dipole moment | 10.1 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 326.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1510 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H319 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Explosive limits | Non-explosive |
| LD50 (median dose) | LD50 (oral, rat) > 2000 mg/kg |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Ethanesulfonic acid 2-(Hydroxyimino)ethanesulfonic acid Methanesulfonic acid Ethylenediaminetetraacetic acid (EDTA) MES (2-(N-morpholino)ethanesulfonic acid) |