Chemists searching for selective electrophilic reagents in the latter part of the 20th century launched the development of trifluoromethanesulfonate derivatives. The potent triflate moiety, famous for its leaving group ability, caught the attention of researchers aiming to create reliable sources for trifluoroethylation. 2,2,2-Trifluoroethyl trifluoromethanesulfonate emerged in laboratories that wanted both a robust activating group and an electron-poor alkyl chain. Not only did these goals suit synthetic organic chemists, but also those tinkering in pharmaceuticals and materials science, who often needed reagents tough enough to withstand harsh conditions, yet reactive in the right hands. The founding work combined sulfur chemistry skills with growing fluoroalkyl demand, so teams quickly pushed this compound beyond the proof-of-concept stage and into catalogues and process batches by the 1980s.
2,2,2-Trifluoroethyl trifluoromethanesulfonate exists as a crystalline or oily liquid, depending on storage and grade. Chemists recognize its striking reactivity, which makes it more than just another building block — it transforms average transformations into uniquely effective couplings or modifications thanks to its powerful electron-withdrawing groups. Pharmacologists might call it intimidating, but seasoned synthesis teams just see another potent alkylating agent that, with the right controls, can boost yields and open doors to new compound classes.
This molecule combines six fluorine atoms with a central sulfur backbone, giving a molecular formula of C3H2F6O3S and a molecular weight near 236 g/mol. It often shows up as a colorless to faintly yellow, low-viscosity liquid, with a boiling point in the 80-90 °C range under standard pressure. The paired triflate and trifluoroethyl groups guarantee notable volatility and strong odor, telling an experienced chemist right away to keep it capped and in well-ventilated spaces. Water splits it quickly, and exposure to common nucleophiles or alcohols sends its trifluoroethyl group flying onto the new substrate. Its solubility in standard organic solvents makes it flexible in multi-step syntheses, but its hydrolytic tendencies mean any experimenter must keep moisture out and lines dry.
From a labeling perspective, the product regularly comes with warnings about irritant and caustic properties, given both triflate and fluoroalkyl groups add hazard. Containers bear GHS hazard pictograms and R/S phrases spelling out what to do after spills or splashes. Analytical details list purity — typically 97% or higher by gas chromatography; density and refractive index round out basic physical data, so buyers can confirm identity and concentration before launching sensitive reactions. As someone who has weighed and diluted these sources, I always check batch history and manufacturing date, since hydrolysis or decomposition sneaks in after only a few months on the wrong shelf.
Synthetic routes for this triflate draw on well-established sulfonation reactions. Most processes start with 2,2,2-trifluoroethanol, which undergoes reaction with trifluoromethanesulfonic anhydride in the presence of a non-nucleophilic base, often pyridine or triethylamine. This step generates heat and acidic byproducts, so all glassware and cooling systems must withstand strong acid conditions. Some industrial setups swap out the anhydride for a chlorinated triflate intermediary, then complete esterification with the alcohol. Careful stoichiometry and staged temperature control make or break yields. Poor laboratory technique or dirty reagents allow exothermic side reactions and lead to messy chromatography later. Any practitioner knows cleanup after a failed batch chews up hours, so following published procedures closely pays off every time.
The most celebrated feature of 2,2,2-trifluoroethyl trifluoromethanesulfonate comes from its dual-activated structure, which primes it for rapid alkylation and esterification. Chemists often use it to introduce the trifluoroethyl group onto oxygen, nitrogen, or sulfur centers. In my own experience, it slots neatly into a variety of nucleophilic substitution reactions, especially those targeting late-stage derivatization of drug-like molecules, where other electrophiles stall. The triflate leaves smoothly, so reaction times shrink and product purifications run easier. Laboratories exploring carbon–fluorine chemistry find it irreplaceable for generating perfluorinated motifs or for tagging sensitive positions prior to spectroscopic tracing. I’ve seen teams in analytical chemistry exploit these same features for labeling small molecules during trace analysis, further underscoring its cross-disciplinary use.
Suppliers and catalogues use a few naming conventions for this molecule, creating some confusion for first-time buyers. Alongside “2,2,2-Trifluoroethyl trifluoromethanesulfonate,” one finds references to “Trifluoromethanesulfonic acid 2,2,2-trifluoroethyl ester,” “TFETf,” “CF3CH2OTf,” or even more compact labels like “TFEE triflate.” Each variant points to the same source, though, so scientists juggling international publications or supplier lists benefit from checking structural diagrams before placing an order.
Lab safety doesn’t leave wiggle room with this compound. Toxic fumes come off quick, and spills corrode gloves and benchtops if not cleaned up immediately. The reagent eats through nitrile quickly; double gloving and using face protection take priority. Local exhausts and fume hoods prove non-negotiable — even small doses in open air sting the nostrils and eyes. Waste management calls for strong base-neutralization before disposal, as the molecule interacts unfavorably with both acidic and basic aqueous streams. Emergency response plans cover direct skin contact and accidental ingestion, as the compound’s reactivity leads to burns rather than just mild irritation. I have seen colleagues overestimate glove integrity and pay the price, so peer-reviewing safety setups before each run becomes standard operating procedure, not optional.
Medicinal chemistry and agrochemical research benefit the most from this reagent’s capabilities. Fluoroalkylation often improves metabolic stability and bioavailability, so introducing trifluoroethyl groups can turn a fragile lead molecule into a drug candidate. Process chemists use it for tuning reactivity in peptide protection schemes or for modifying natural products, especially when existing methods fall short. Organic materials scientists wish to load polymers or surfaces with fluorinated chains, and this reagent allows precisely that through surface alkylation. As a former bench chemist on interdisciplinary projects, I relied on it not only for efficiency but also for the ability to avoid more cumbersome protection–deprotection sequences that less selective alkylating agents demand. Beyond pharmaceuticals, researchers running systematic structure–activity relationship studies see better clarity in how the trifluoroethyl handle influences biochemical pathways, thanks to this direct and robust alkylating action.
Research teams keep finding new places to slot this reagent into reaction schemes. Ongoing projects in the past decade pushed its utility as a radiolabeling precursor and explored greener, less acidic process modifications. Some pharmaceutical groups look into its enantioselective deployment, using it to separate or resolve complex mixtures with chiral auxiliaries. Environmental analysts examine its breakdown in soil or aqueous environments, looking for evidence of hydrolysis products or any unanticipated reactivity. Materials groups employ high-throughput screens to expand applications in fluoropolymer modification, investigating both bulk and surface properties. From personal observation, even smaller-scale contract research labs devote resources to refine purification and storage methods, driven by client demand for high purity building blocks that withstand tough late-stage transformations.
Toxicology data on 2,2,2-trifluoroethyl trifluoromethanesulfonate continues to be collected, but what is known already signals caution. Acute exposure leads to mucous membrane discomfort and may cause delayed tissue damage, given both the acid-forming breakdown products and the fluoroalkyl fragments. Inhalation studies flag it as a respiratory hazard, with animal models showing inflammation and, after repeated exposure, significant risk to liver or kidney function. Chronic low level exposure impacts metabolism and can disrupt enzymatic pathways, much like other highly fluorinated compounds. Safety guidelines demand strict personal protection and limit handling to properly ventilated and monitored environments. As green chemistry principles spread, researchers look for less toxic alternatives, but until equally reactive options exist, only disciplined safety culture shields experimenters.
Ongoing innovation still circles around the promise and peril of this triflate. Demands for more sustainable, less hazardous fluorination reagents charge ahead. The focus shifts toward designing derivatives that combine high activity with easy degradation or faster environmental breakdown. Emerging trends point toward automated synthesis platforms that use microfluidics and contained systems for short-lived intermediates, reducing both waste and operator exposure. As regulations on perfluorinated substances evolve, those producing or using this reagent will have to track changing compliance landscapes. Green chemistry hopes to either tame or replace these powerful, sometimes unwieldy, tools. Still, for anyone solving complex molecular design problems, 2,2,2-trifluoroethyl trifluoromethanesulfonate remains a steadfast ally, at least until the next innovation truly challenges its versatility and reliability.
2,2,2-Trifluoroethyl trifluoromethanesulfonate, sometimes dubbed “TFETf,” brings a very specific skill set to the chemistry toolkit. Labs turn to this chemical for jobs that need a strong kickstart, particularly in the birth of other molecules. Having worked in a research setup, I’ve watched how this compound unlocks doors that other reagents cannot quite budge. It carries both a highly reactive leaving group and a trifluoroethyl ingredient. That means it can slip onto molecules where less ambitious chemicals balk, pulling off transformations that make or break a synthesis project.
TFETf steps in mostly during the installation of a trifluoroethyl group. The trifluoromethyl family produces effects that organic chemists crave: stronger metabolic stability, resistance to breakdown, and sometimes added resistance to heat or acidity. These features help when a pharmaceutical molecule needs a trifluoroethyl tag to boost performance inside the body. I’ve seen synthesis teams wrestle with older methods, only to switch to TFETf as a cleaner, more dependable route. The molecule’s Reactivity, especially under mild conditions, reduces time wasted on harsh acids or high temperatures.
Medicines increasingly include fluorine due to its ruggedness and small size, and TFETf acts as a shortcut in attaching trifluoroethyl arms. Drug discovery doesn’t really have the luxury of slow reactions, so chemists favor reagents that cut complexity. In many projects, researchers rely on TFETf to build blocks that could fight inflammation, attack cancer, or control blood sugar. Its cousin applications emerge in crop protection. Pesticide and herbicide teams often chase greater selectivity, hoping to ward off pests while sparing more desirable plants. Adding trifluoroethyl arms with TFETf sometimes shields agrochemicals against degradation, leading to longer-lasting field results and even reducing how much chemical ends up released into the soil.
Chemists don’t reach for TFETf lightly. It reacts quickly and doesn’t forgive sloppy technique. Anyone working with it suits up with gloves and eye protection. Left uncapped, TFETf can irritate airways and skin or cause burns if splashed. I’ve trained new lab members on TFETf handling, always stressing strict adherence to safety protocols and good ventilation, as per recommendations from major safety organizations like OSHA and the American Chemical Society.
Demand for greener chemistry keeps rising. TFETf’s potent properties sometimes overshadow its environmental baggage. Disposal introduces challenges, and traces in waste streams can linger. As someone who’s been tasked with chemical inventory decisions, I’ve followed the hunt for alternatives. Some researchers explore milder fluorinating agents, or tweak catalytic systems for similar results but lower environmental cost. Others scope out process improvements—closed loops and advanced purification steps—to cut the chemical waste load. Policy shifts may eventually steer chemists away from certain reagents, TFETf included, nudging the entire field toward safer, cleaner practices.
TFETf brings a rare combination of raw power and selectivity. It helps build advanced materials and medicines, but it also challenges its users to respect its limits. Chemistry teachers, safety officers, and project leaders all share a role in making sure this tool gets used wisely and sparingly. Responsible application can keep the benefits flowing without tipping the scales toward greater risk or waste.
Most people don’t give much thought to the risks behind a bottle in the chemical storeroom, yet every label tells a story worth listening to. 2,2,2-Trifluoroethyl trifluoromethanesulfonate brings clear danger. The main threat shows up as severe skin burns, respiratory irritation, and eye damage. Inhalation or skin contact can hit hard, quickly. No one enjoys a chemical surprise, especially when it can punch through gloves or turn a brief slip-up into a hospital visit.
Real lessons stick when accidents get close to home. A seasoned chemist once showed me her hands, scarred from one careless afternoon with corrosives. Stories like hers drive home the need for real protection. Splash goggles block fumes and droplets from finding your eyes. Lab coats shield clothes and skin, but the job demands more—high-quality nitrile or butyl rubber gloves have to be swapped when they get contaminated. Taking shortcuts with gloves only looks like it saves time.
Good airflow makes a direct difference. Using this compound in an open workspace turns a routine procedure into a guessing game—who will cough first, who gets headaches? By working in a chemical fume hood, air pulls any vapor away from your breathing space, proving every safety poster right. Fume hoods also contain splashes, reducing chemical spread. It’s about respect for those working nearby, not just yourself.
You don’t want to grab a bottle and discover it leaked overnight. Store this material in tightly sealed glass containers, well away from heat, sunlight, and sources of ignition. Flammable solvents and acids should sit on separate shelves. Water eager to react with trifluoroethyl trifluoromethanesulfonate lurks in the air, so desiccators and dry environments keep things calm. Resisting the urge to top off dusty bottles limits unnecessary exposure. Tall shelves have no place in this routine: an easy reach at eye level means fewer drops and spills.
Too often, labs overlook the placement and maintenance of eyewash stations and safety showers. Having these within arm’s reach turns a potentially disastrous spill or splash into an inconvenience instead of an emergency. Practice reaching for safety showers and locate the nearest exit before opening a bottle. It makes all the difference when seconds tick away during a panic.
Training sessions sometimes feel tedious, but the little reminders stick—the rush to clean up every drop, the check for glove holes, the double-check before opening a bottle. Habits form from these small rituals, and each step works as a barrier against the most painful mistakes. I’ve watched new lab members shadow veterans, picking up the unseen tricks that don’t make it into safety manuals. Learning to ask about proper waste disposal instead of guessing, or calling for backup when a reaction looks wrong, always pays off.
It’s easy to focus on individual habits and miss bigger problems. Institutions do well to update their chemical hygiene plans, run unannounced drills, and encourage reporting of near-misses. Strong leadership goes beyond handing out gloves—it means making the team comfortable enough to speak up when shortcuts tempt them. New safety technology, from better glove materials to improved fume hood designs, deserves attention during every safety meeting. Every layer counts.
2,2,2-Trifluoroethyl trifluoromethanesulfonate plays a unique role in synthetic chemistry, serving as a potent alkylating agent. Through years spent working alongside seasoned chemists, I’ve seen what can go wrong when storage of reactive reagents takes a back seat to convenience. This compound isn’t just flammable—it’s also moisture sensitive and capable of generating toxic byproducts. That means tossing it into a generic chemical cabinet spells trouble.
Laboratories rarely run perfectly dry, so water vapor lingers everywhere. 2,2,2-Trifluoroethyl trifluoromethanesulfonate reacts with moisture, and the byproducts can corrode containers or harm workers nearby. During my stint at a research institution, a few drops of water leaking from a faulty refrigerator led to a ruined bottle and unexpected downtime. We learned, sometimes painfully, that storing this chemical in tightly sealed glass bottles pays off in spades. Using plastic risks softening or even punctures through slow reaction with the chemical.
Every major chemical safety resource calls for cool storage, but this advice matters even more with this trifluorinated reagent. Leaving the bottle on a warm benchtop lets vapors build up, increasing the chance of fume inhalation or leaks. Cold, dry conditions inside an explosion-proof fridge—in a lower shelf, well away from the fan unit—keep degradation at bay. Wherever I worked, labeling the storage spot in the fridge with a laminated card helped keep new team members clear on the rules. Spill trays under every container caught any accidental leaks, sparing us stressful cleanups.
There’s always pressure to save time in the lab. But cutting corners on labeling burns trust and slows investigations if something goes wrong. Each bottle should display the chemical’s name, date received, date opened, and the initials of the handler. That simple habit saved me from using expired stock on more than one late-night synthesis run. Keeping 2,2,2-trifluoroethyl trifluoromethanesulfonate away from acids, bases, and oxidizers reduces risk of runaway reactions. Segregating reactive chemicals in separate secondary containers turns a cluttered fridge into a safer, more navigable workspace. Team training on these habits, reinforced by posters and checklists, builds a strong community of safety.
High-value reagents, especially ones with acute hazards, call for sign-in logs and restricted key cards. Some facilities assign responsibility for specific cabinets, and that layer of ownership helps keep policies on track. Regular inspection—weekly or at least monthly—catches leaks or corrosion long before they escalate. I recall swapping out a worn gasket on a dusty bottle just in time to avoid disaster. A culture of vigilance lowers incident rates, as proven in academic safety reviews and Responsible Care reports from the chemical industry.
Proper storage of 2,2,2-trifluoroethyl trifluoromethanesulfonate isn’t about bureaucracy, it’s about health and results. Investing time in routine checks, labeling, and using the right containers under dry, cool conditions keeps both people and experiments out of trouble. Smart lab management makes great discoveries possible—and with vigilance, these tough lessons only have to be learned once.
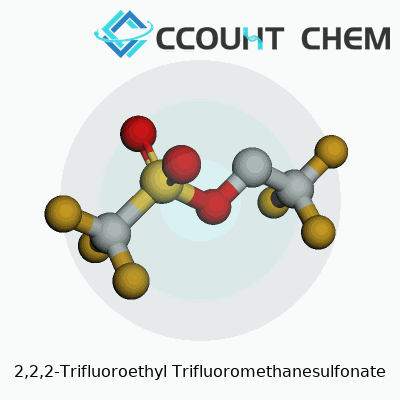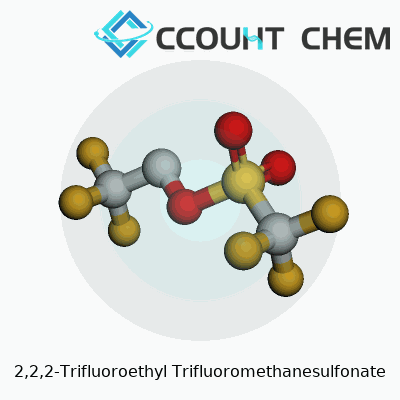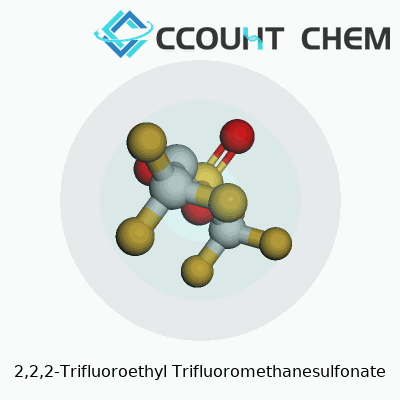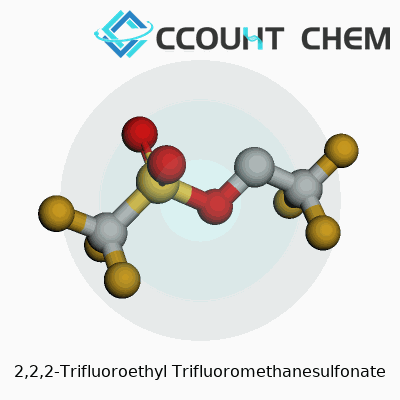
Scientists and engineers want information they can rely on. If you’re getting into the world of organofluorine chemistry, 2,2,2-Trifluoroethyl trifluoromethanesulfonate matters. To put it plainly, the chemical formula for this compound is C3H2F6O3S. Its molecular weight clocks in at 236.10 grams per mole, a figure trusted by labs and referenced in peer-reviewed journals. This formula captures the unique structure—a three-carbon backbone flanked with fluorines and capped with the ever-aggressive trifluoromethanesulfonate group, often known as “triflate.”
In my early days handling reagents, I didn’t appreciate the draw of perfluorinated molecules. The triflate group changes everything. It’s not just a decorative feature—the triflate makes a molecule much better at leaving during a reaction, opening the door for building complex molecules. Chemists pick 2,2,2-Trifluoroethyl trifluoromethanesulfonate for its powerful leaving group performance. The unique mix of a trifluoroethyl side chain and a triflate transforms reaction outcomes, especially for substitutions or alkylations where a so-so leaving group just won’t do.
You’ll find this compound on the shelf of any medicinal chemistry or advanced organic synthesis lab. Drug designers value it for inserting fluorine-rich motifs that can improve the metabolic stability of new medicines. These groups tend to stick around in the bloodstream longer, a fact supported by countless pharmacokinetic studies. Fluorinated esters like this also pop up in agrochemical pipelines and material innovation, as the demand for special performance coatings and electronic materials keeps growing.
I remember the caution in my supervisor’s voice while handling strong electrophiles. 2,2,2-Trifluoroethyl trifluoromethanesulfonate doesn’t mess around. It reacts rapidly with nucleophiles, which gives it utility but also requires responsible handling. Safety data points to the risk of severe eye and skin irritation. In well-run labs, technicians suit up with gloves and goggles, work with good ventilation, and keep neutralizing agents on hand in case of spills. Material safety data sheets spell out the facts without sugarcoating the risks.
Cost and environmental impact fuel debates about whether labs should choose reagents like this. Fluorinated organics linger in water and soil, so waste disposal calls for more than a trip to the sink. Studies from environmental chemists warn about the persistence and bioaccumulation of perfluorinated compounds. Labs adopting green chemistry principles push toward alternatives when possible—choosing less hazardous sulfonates or tuning reaction conditions to minimize leftover waste. Some universities set up waste tracking systems for every fluorinated bottle, linking disposal records with purchasing histories to audit and refine protocols.
2,2,2-Trifluoroethyl trifluoromethanesulfonate, with its formula C3H2F6O3S and weight of 236.10 g/mol, isn’t just another reagent in the cabinet. Its impact stretches from the bench in academic labs to large-scale pharma production lines. Familiarity with its properties and risks gives researchers and engineers the tools to use it wisely and safely—something every serious maker of molecules should strive for.
2,2,2-Trifluoroethyl trifluoromethanesulfonate, sometimes called a “triflate” in the lab, packs both reactive and stable qualities. The three fluorines on the ethyl group make it less likely to react uncontrollably, but the triflate group itself brings real punch—strong enough to flip even simple alcohols into excellent leaving groups. In my chemistry days, few reagents got as much respect for their efficiency and neat tricks in synthesis. At the same time, its extreme reactivity draws some red lines in what you can mix with it.
Dry and non-nucleophilic solvents show the best results, like in so many reactions where you don’t want unexpected side products. Dichloromethane, acetonitrile, and toluene allow the reagent to do its job. When acetonitrile is dry and fresh, it lets the triflate swap groups cleanly—my colleagues and I leaned on it, especially when moisture wrecked things.
Water and alcohols change the outcome. Even low levels of water might hydrolyze the reagent, wrecking yield and creating a mess. Ethanol or methanol tend to jump in and react, leading to unwanted byproducts. Most who run these reactions keep everything dry, check bottles, and toss any hint of cloudiness. I’ve seen a careless rinse add enough water to turn a great triflation into a disaster. This isn’t just textbook caution—it saves hours of wasted effort.
Nucleophiles can act unpredictably. Amines, for example, go after the triflate fiercely. With strong or basic nucleophiles, things happen too fast—uncontrolled, messy, wasteful. In crowded labs, someone eventually tries to shortcut the process with cheap bases or “faster” stirring. More often than not, the result is either ruined product or safety worries from strong, unpleasant fumes.
Lithium, sodium, and potassium-type bases sometimes cause exothermic reactions. If you’ve sat near an experiment that gets suddenly hot, you learn to treat combinations with suspicion. You don’t forget the sharp smell or the dash for the fire blanket. Safer options involve mild, buffered conditions with weaker bases like pyridine—these approaches produce smoother, cleaner outcomes and let you actually isolate what you want.
Sunlight, heat, and open air are serious enemies for this reagent. Kept cold and in tightly sealed, dark bottles, it stays quiet; left unattended on the bench, it begins breaking down, wasting cash and time. One summer, air conditioning failed in a storage room—a few bottles lost their clarity, and tests showed breakdown products. Those moments remind every chemist why you read handling instructions twice and treat waste with caution.
The best chemists plan ahead. They check for water, dry the glassware, and monitor reactions. Using glove boxes or Schlenk lines cuts contamination risks and boosts success. Chemically resistant materials—Teflon, glass—hold up under these conditions, where rubber or plastic joinings might degrade.
Industry leaders share experience and rigorous records, highlighting batch issues or surprises with different grades of solvents. These stories keep younger scientists from repeating avoidable mistakes and ground big discoveries in real-world prudence.
Real progress doesn’t just mean faster reactions. It grows out of understanding dangers and oddities that come only with regular practice—being willing to test, double-check, and share not just successes, but failures. With 2,2,2-trifluoroethyl trifluoromethanesulfonate, reliability always starts with clean, compatible solvents and a healthy respect for all the sneaky ways this molecule can surprise you.
| Names | |
| Preferred IUPAC name | 2,2,2-trifluoroethyl trifluoromethanesulfonate |
| Other names |
2,2,2-Trifluoroethyl triflate Trifluoromethanesulfonic acid 2,2,2-trifluoroethyl ester 2,2,2-Trifluoroethyl methanetrifulfonate Trifluoroethanol triflate |
| Pronunciation | /ˈtraɪˌfluːəroʊˈɛθɪl ˌtraɪˌfluːəroʊˈmɛθeɪnˌsʌlˌfəˌneɪt/ |
| Identifiers | |
| CAS Number | 431-55-2 |
| Beilstein Reference | 881478 |
| ChEBI | CHEBI:86963 |
| ChEMBL | CHEMBL4158673 |
| ChemSpider | 22595700 |
| DrugBank | DB08370 |
| ECHA InfoCard | 03c590d2-0e71-4576-b1e8-4340b790baf6 |
| EC Number | 212-607-6 |
| Gmelin Reference | 89847 |
| KEGG | C18752 |
| MeSH | D017325 |
| PubChem CID | 73820 |
| RTECS number | YT5425000 |
| UNII | C1VV58361L |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID2021851 |
| Properties | |
| Chemical formula | C3H2F6O3S |
| Molar mass | 284.13 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet odor |
| Density | 1.594 g/mL at 25 °C (lit.) |
| Solubility in water | Decomposes in water |
| log P | 1.7 |
| Vapor pressure | 0.4 mmHg (20 °C) |
| Acidity (pKa) | pKa ≈ –12 |
| Magnetic susceptibility (χ) | -46.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.297 |
| Viscosity | 1.06 cP (20°C) |
| Dipole moment | 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –1103.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1649.1 kJ/mol |
| Pharmacology | |
| ATC code | This product does not have an ATC code. |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P260, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 Reactivity:0 Special: |
| Flash point | 14 °C (closed cup) |
| Autoignition temperature | 225°C |
| Lethal dose or concentration | LD50 (oral, rat): 300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 206 mg/kg |
| NIOSH | TTT19200 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Methanesulfonic anhydride Trifluoromethanesulfonic anhydride Ethyl trifluoromethanesulfonate Nonafluorobutanesulfonic acid Trifluoromethanesulfonic acid |