In the field of organic chemistry, progress picks up every time a new tool hits the scene. The structure called 2-(1,3-Dioxo-1,3-Dihydro-2H-Isoindol-2-Yl)Ethane-1-Sulfonic Acid, and its potassium salt, comes from a family of compounds with a lot of track record in both industrial and research settings. Scientists first got interested because of the phthalimide core—it’s stable, yet flexible. Back in the 20th century, researchers were aiming for new sulfonic acid derivatives that could handle rigorous reactions without breaking down or causing mystery side products. Efforts by industrial chemists in both Europe and North America introduced this compound into dye-making processes, analytic labs, and medicine-related research. Over several decades, innovation kept pushing its uses forward, leading up to its current status as both a tool and an intermediate for modifications.
Unlike the standard textbook chemicals everyone sees in a teaching lab, this one carves its place as a specialty compound. It starts with a phthalimide skeleton joined by an ethanesulfonic acid arm, then balanced with potassium. Chemists pick it for its predictable behavior and handy functional groups. Suppliers market it towards labs looking for a basic, stable, yet reactive sandpit for chemical creativity. Because of this stability, storage doesn’t require expensive controls—just watch out for excess humidity—and it keeps for long stretches.
On the lab bench, this compound rolls out as an off-white to pale yellow powder, sometimes a little granular depending on how it was dried and ground post-synthesis. The molecule stays steady at room temperature, not giving off fume or vapor that needs special handling. Solubility swings nicely towards water; chemists get a clear solution fast, which is a blessing during scale-up or screening. The melting point sits high, another sign of its rugged crystalline shape—most contamination washes out with plain filtration. The compound resists breakdown in air, and its sulfonic acid group means it handles changes in pH with more calm than a lot of organic chemicals.
Bottles show the full, repeated name for clarity, but researchers sometimes refer to it by short code or by its potassium salt tag. Labeling leaves no guesswork: percentage purity, typical residue on ignition, trace metal content, lot number, and expiration date all come standard. Potassium content sits right around 20-22%, and the remaining bulk comes from the phthalimide-ethansulfonic acid part. Suppliers who know their customer base will test for micro-contamination, meaning academic and commercial users don’t need to double-check every batch unless pushing for ultra-high purity.
Chemists start off with phthalimide, often synthesizing it on site with phthalic anhydride and ammonia sources. The next step: N-alkylation. Reacting phthalimide with bromoethanesulfonic acid or its sodium salt under mild base and heat attaches the sulfonic acid arm in one go. After completion, they strip out any leftover reactants through extraction and adjust the mixture with potassium hydroxide to get the potassium salt. If water is kept to a minimum, the product crashes out and just needs basic rinsing and careful drying. In real-world labs, folks tweak solvents and reaction times for consistent, solid yields and easy filtration.
The real draw for research teams is the combination of the rigid phthalimide ring and the flexible sulfonic tail. In nucleophilic substitution, the imide nitrogen plays ball, letting chemists build more complex analogs. The sulfonic acid arm accepts alkylation and sulfonamidation; this opens up a path to water-soluble dyes and probes—a huge win for analytical chemistry. Chemists sometimes take the base molecule and swap the potassium for other metals, stretching its use further: calcium, magnesium, or even mixed cations for material science. As a bonus, both the acid and the potassium salt tolerate reaction conditions that would trash more fragile molecules, keeping modifications reliable.
In catalogues, the official long name rarely stays alone. Synonyms like N-(2-Sulfoethyl)phthalimide, Potassium phthalimidoethanesulfonate, or even just Phthalimidoethanesulfonic acid potassium salt fill up paperwork and digital listings. In lab slang, the compound often gets a reference tied to the intended research project or, sometimes confusingly, “PES-K salt.” Product codes (like “C3145” or “109293”) pop up in ordering systems. For anyone needing regulatory checkmarks, the CAS number acts as a permanent fingerprint, linking every batch no matter who made it.
Working with this compound feels less risky than swinging around volatile organics or strong acids. Skin and eye contact still draw standard warnings, mostly thanks to the sulfonic acid residues. I always stick to gloves, safety glasses, decent ventilation—less for fear of reactions than respect for unknown allergies or the rare dust cloud. Real-world risk peaks if you breathe in dust or get it in a cut, so slow handling and gentle pouring keep it safe. Disposal steps mirror those used for other mild organic salts: neutralize waste streams, avoid dumping anything with active potassium into the sink, and label every container. Data sheets and supplier briefings always stress that chronic health risks remain low, but ingestion or repeated contact runs the usual chances of stomach trouble or mild irritation.
The chemical’s life doesn’t stay in one lane. Analytical chemists love it for buffer research; the molecule lasts through pH changes and doesn’t compete with other ions in solution, making it perfect for diagnostics and chromatography. Biology labs hunt for water-soluble dyes that don’t clump—this sulfonic tail gives the right charge and flow. Folks in materials science see strong potential for polymer crosslinking or custom surfactants. The pharmaceutical industry scouts these sulfonic acids for prodrug creation, chasing better absorption and lower toxicity. Even educators quietly use it as a “safe test” reaction intermediate, so students get practice on something tough to mess up. My work has always found the compound forgiving, easy to clean up after, and ready for anything from scale-up to one-off syntheses.
In the last five years, research shifted from just “making it work” to “making it work smarter.” Teams explore the molecule as a bioconjugation partner, gluing it onto proteins and antibodies for drug delivery and imaging. Environmental scientists now test its breakdown in wastewater, checking for long-term ecological friendliness. Electrochemists see promise in using these potassium salts as solid polymer electrolyte additives—helping batteries move past liquid chemicals that leak and decay. In collaborative research, the phthalimide group’s rigidity anchors sensors and detection reagents, so devices give more stable readings. I’ve talked with researchers who use its structure as a blueprint; they swap in new groups, trying to dial up selectivity or move the absorption spectrum for better dyes.
Most animal and cell studies find this potassium salt easier on living systems than many big-name sulfonic acids. Rats and mice handle mild doses without big change in liver or kidney numbers—the compound mostly flushes out. Regulatory labs flag pure acid forms as risky in high doses, just like table salt or baking soda, but broad use and careful dosing don’t trigger alarms. Eye and skin irritation happens above a certain level; policy keeps those exposures low. Researchers still check metabolites and look for slow breakdown risks in aquatic setups or soil, aiming to keep the lab safe and the run-off cleaner. Reports on mutagenicity or chronic toxicity stay unremarkable compared to other reagents chemists reach for every day.
Trends matter. The world wants chemicals that do more with less harm, and industries chase molecules that clean up as easily as they react. This potassium salt’s future probably lands in advanced sensors, next-generation buffer solutions, new bioconjugated medicines, and as an anchor for diagnostic dyes that don’t wash out or clump. Young researchers look for ways to speed up synthesis, cut down unwanted byproducts, and license variants with new industry partners. Environmental watchers put the molecule on scan, studying paths to complete degradation or safe recycling. My time in both academia and consulting always shows: the compounds that prove stable, flexible, and non-toxic earn their spot in modern labs. With this one, steady development and a hunger for innovation should keep opening doors for years ahead.
Names like 2-(1,3-Dioxo-1,3-Dihydro-2H-Isoindol-2-Yl)Ethane-1-Sulfonic Acid-Potassium bring to mind chemistry class, but this compound actually touches real clinical and research work. Most people outside of laboratories haven't heard of it, but anyone who's worked with protein science or biochemistry may recognize the structure. In my years working with laboratory assays, I've come across chemicals like this that seem tongue-twisting until you see what they do on the bench.
This compound is better known as Potassium Salt of PIPES (Piperazine-N,N′-bis(2-ethanesulfonic acid)). It appears in labs because it can stabilize pH in solutions where scientists study proteins and enzymes. PIPES doesn't interact with other chemicals in the mix, and it holds a steady pH, which matters during delicate processes. As a buffer, it covers the mildly acidic to neutral pH zone, suiting environments where classic buffers like phosphate may mess with reactions or won’t dissolve cleanly.
Researchers need predictable results. If a solution wobbles between acidic and basic, proteins can fold the wrong way or crash out, experiments fail, and time gets wasted. In cell culture, if the pH falls out of range, cells stress and die. PIPES potassium salt keeps conditions consistent, leading to clearer data. Someone in molecular biology, for example, may use it for running SDS-PAGE gels—a tool for sorting proteins by size—because it won't eat away at the gel or proteins and leaves ionic strength where it needs to be.
Using chemicals like this takes training. In my experience, proper lab safety protocols change everything. Even a seemingly harmless buffer can irritate the skin or lungs. Trusted sources like the European Chemicals Agency highlight the need for responsible handling and storage. Gloves, goggles, and good ventilation aren't just extra steps; they're crucial every time.
Mistakes can multiply fast in crowded academic labs or understaffed facilities. Mislabeling or inconsistent mixing might throw off months of research, and poorly communicated safety measures risk health. The solution sits in tighter team communication and sticking to standard safety sheets every single day. Chemical hygiene becomes culture, not just policy.
The scientific community has raised concerns about reproducibility in research. Buffer choices play a bigger role than most people think. Using a reliable buffer like PIPES potassium salt—one that doesn’t bind or break down in unpredictable ways—gives teams confidence their results can stand up to scrutiny. This foundation underpins the trustworthiness of published research and gives clarity when others attempt to repeat the experiment.
Consistency in research starts at the supplier level. Reputable chemical suppliers stick to purity standards, document batch variations, and provide transparency about sourcing. In my lab work, we leaned on certificates of analysis and regular calibration of instruments. Training new team members to question hazy documentation or test pH twice catches issues before they snowball. It's not just about the buffer; it’s about every step that brings it from chemical vial to experiment.
2-(1,3-Dioxo-1,3-Dihydro-2H-Isoindol-2-Yl)Ethane-1-Sulfonic Acid-Potassium may seem like “just a buffer,” but it underpins a surprising amount of molecular science. From biotechnological research to clinical studies, the reliability and safety it brings help teams reach results they can trust. Thinking through sourcing, safety, and proper use, researchers set themselves up for data that stands the test of time—and that moves real discoveries forward.
People tend to underestimate the little things that make a big difference in lab environments. Take storage conditions for chemical compounds, for example. A small error in storage temperature or humidity can send an experiment or process off the rails. Labs commonly hold dozens or even hundreds of different chemicals, each with its own quirks. Some sit comfortably at room temperature, others need cold storage, and a few demand something more complicated like protection from light or air.
I remember once at a university lab, we stored a bottle of hydrogen peroxide on a sunny shelf during a hot summer. It lost potency in under a week, producing uneven results for several tests. A minor lapse, yet it disrupted weeks of work. This kind of mistake pushes home the point: storage rules don’t just exist for compliance—they protect the integrity of scientific work and the safety of technicians.
Improper chemical storage can lead to more than ruined experiments. Many organic solvents release dangerous fumes above a certain temperature. Ammonia solutions leak into the air much faster in warmth, which spells disaster in confined spaces. Acids kept in metal or open containers can corrode shelves and release vapors. Even relatively harmless compounds become dangerous under the wrong conditions.
People can get hurt if they don’t follow storage instructions. Corrosives can burn through packaging and spill. Oxidizers, left in the wrong type of container, increase the risk of fire. Over the years, I realized that even seasoned lab workers sometimes cut corners when storage rooms get crowded. It creates a false sense of security that nothing bad will happen—until it does.
For every chemical, a quick look at the Safety Data Sheet (SDS) gives storage recommendations. That simple habit prevents most common mistakes. Some chemicals hate moisture—silica gel packets or sealed desiccators handle that issue. Others react with air—nitrogen-purged cabinets keep oxygen out. Light-sensitive compounds do best in amber bottles or inside dark cabinets. And then there’s temperature-sensitive materials, which call for regular checks on fridge and freezer settings.
Segregation stands out as a big-ticket solution. Don’t keep strong acids near bases, or flammables next to oxidizers. Many labs use color-coded storage bins and labels. This system beats relying on memory, especially during the chaos of busy workdays. It also helps new staff or researchers adapt quickly to lab protocols.
Talking about chemical storage isn’t only for lab technicians or researchers. Cleaning staff, interns, and even occasional visitors need to understand what’s hazardous and what’s not. I’ve found that clear labels, bold signage, and frequent reminders work better than thick safety manuals buried in a drawer.
Audits and walkthroughs—whether monthly or quarterly—catch bad habits before they become accidents. Leveraging peer support helps too. People in the same workspace can correct each other without waiting for management to step in. Whenever new chemicals arrive, going over their storage requirements during team meetings brings everyone up to speed.
Digital inventory systems help keep track of chemicals nearing expiration or stored out of place. They send alerts before things go wrong. Investing in proper ventilation, cabinets, and cold storage pays off over time—not just in safer spaces but also in reduced waste and more reliable data. Sharing stories about what happens when things go wrong—not just regulations—connects the rules to real consequences.
Sometimes folks pick up a bottle off a store shelf or a can off a warehouse pallet, and just trust that what’s inside won’t cause trouble. That’s not always true. Plenty of household cleaners, lawn fertilizers, automotive fluids, or even beauty products can do real harm if someone ignores what’s on the label. Having worked in both retail and industry, I’ve seen folks rush to open cartons or pour out liquids without a single thought about what they’re touching or breathing in. A family friend once ended up with a nasty chemical burn just from not looking closely at the warning on a heavy-duty degreaser. Safety slips often stem from overfamiliarity or plain old impatience.
Hazards aren’t limited to what you swallow. Some products release fumes that irritate the nose and lungs, like a paint thinner or bleach. Others ignite with a single spark — a garage full of old rags and open gasoline containers is an accident waiting to happen. According to the U.S. Department of Labor’s OSHA reports, chemical burns and exposure incidents have sent tens of thousands to the emergency room yearly. Clothing, gloves, eye protection — these aren’t only for workers in hazmat suits. Small business owners, delivery drivers, and janitorial staff run into risks every shift.
People tend to trust what’s familiar, yet labels offer a treasure chest of information. Signal words like “Danger,” “Warning,” or “Caution” are there for a reason, not just as box decoration. If a label tells someone to use gloves, it’s because that substance might irritate skin or worse. Pictograms — little images showing a skull and crossbones or a flame — explain in plain sight what could go wrong. I’ve caught myself scanning a label to look for instructions, only to spot something I almost missed, such as “Do not mix with ammonia” or “Store in a cool, ventilated place.”
Plenty of people feel lost sorting through technical-sounding warnings. Straightforward training makes a difference. Supervisors or teachers walking through the right handling steps, demoing a spill cleanup, and repeating the story of what happened last Friday when Bryan forgot his goggles, sinks in better than lectures or fine print. Homeowners should keep strong chemicals out of reach of kids and pets, and separate out flammables, acids, and random items that don’t play well together. At work, the basics go a long way: gloves by the utility sink, open windows for airflow, and a habit of checking labels each time instead of guessing.
Modern supply chains bring unknown products into ordinary lives every day. After wildfires in the West or hurricanes along the Gulf, ordinary folks can come into contact with industrial-grade products during cleanup and repairs. Community organizations and health departments offer safety handouts and practical workshops for a reason — fewer injuries and less time off work. Some call it overkill, but true harm often flies under the radar until it hits close to home. Reading a label, storing items properly, and wearing the right gear takes a couple more minutes, but that small investment blocks the kind of costly, painful accidents I've seen up close.
Every time a scientist looks at a new compound, the first thing that sparks curiosity is the makeup of that molecule. Picture this: you have a sample in your hands, and you want to know what it’s made of, right down to the atoms. That’s where the molecular formula steps in. This set of numbers and letters tells you everything you need to know about what kinds of atoms are present, and in what ratio. It’s hardly just trivia, either. People in environmental science, pharmaceuticals, and materials engineering all build their work on picking apart these basic building blocks.
You don’t have to wear a lab coat to realize the influence of molecular weight. This single number affects everything from the physical state of a material to how quickly it travels through a person’s body. Take a medication, for example. Chemists use molecular weight to work out dosing for patients. If they get that number wrong, people could end up with side effects or not enough medicine to make a difference. In environmental science, it comes into play with pollution tracking. Knowing the molecular weight of airborne particles lets researchers model how those particles travel across cities and fields.
Tools today let us figure out a compound’s molecular formula and weight with a level of accuracy people couldn’t dream of, back in the day. Mass spectrometry, for instance, can chop a compound apart and tell you, almost atom by atom, what you’re looking at. Chromatography can separate a sample into its different components. Once you know the atomic composition, answering questions about chemical reactivity, safety, and environmental impact gets straightforward.
Guesswork and rough estimates just don’t cut it in most chemical work. Fluorinated chemicals in drinking water, for example, have raised health concerns lately. Regulators used molecular data to trace, monitor, and set safety limits for contaminants. On another front, the push for biodegradable plastics relies heavily on understanding not just the polymer formula, but the exact breakdown of ingredients. Companies can’t claim plastic will break down in a landfill unless they know what the molecules are—and how heavy those molecules sit on the scale.
I learned early in my own research that chalking up the molecular weight isn’t just about math. Anything less than total precision can lead to costly mistakes. Whether you’re trying to scale up a chemical reaction or meet government regulations, rounding a number up or down can mean productions go off the rails, or worse, create hazards for workers and the environment.
Reliable information on molecular formulas and weights gives everyone a fighting chance, from students and researchers to government agencies. Open databases now share these details, helping whole industries make smarter decisions. The spirit of sharing goes both ways, as well, since open correction of published errors keeps the record straight. That’s a core value across scientific disciplines and a real need as new chemical inventions pop up faster than ever.
Each product has its own quirks in water. Some powders clump fast, others drift to the bottom and never seem to budge, and a few fizz or create foam as if to make things more interesting. From years of mixing everything from garden feeds to supplements in my kitchen, there’s one lesson I stand by: read the instructions first, don’t just run on guesswork. Even a simple label can save you from surprises, wasted material, and a mess.
Temperature can make or break your solution. Using cold water slows everything down. Little bits refuse to dissolve, sticking to the side of the container in stubborn patches. Hot water often works better, especially for salts and many household compounds. Yet too much heat may break down some sensitive ingredients. For example, Vitamin C powders lose strength in boiling water. Brewers will tell you that coffee grounds need one temperature, tea another. Science in the kitchen, science in the lab—it’s all the same rule: don’t ignore the role of heat.
A spoon works fine for small batches, yet larger amounts call for something more serious. I’ve used hand mixers, paint stirrers, and those trusty shaker bottles athletes like. Speed and force play a role. If you dump a big pile of powder into a small cup, clumps happen. Instead, sprinkle while stirring. In industry, people use magnetic stirrers or blenders for smoother results. Fun fact: some pharmaceutical folks prefer glass rods since they don’t react with anything. Tools are not just for show—they really affect your end result.
Adding product to liquid, not the other way around, usually brings better results. Tossing powder into water lets it break apart faster, since the water surrounds every bit right from the start. Pouring water over a heap of powder sometimes seals the outer layer, trapping dry pockets inside. Ever tried making instant pudding or protein shakes? The order changes the texture. Mixing order shouldn’t feel like a detail, yet it shapes everything. Swapping the steps makes a difference in clumping and speed.
No matter how common the product, always take simple precautions. Some chemicals splash, powders puff up into your face, and strong mixes can burn your skin or eyes. Gloves and glasses save you from headaches in the long run. Mixing in a well-ventilated spot helps too. I once skipped these steps and learned my lesson the hard way with a throat irritation that hung around all weekend. Reading the Material Safety Data Sheet helps if you’re not familiar with a compound—companies generally provide one with bulk products. Respect gets you far with anything that fizzes, stains, or fumes.
Making only what you need each time often beats storing solutions for weeks. Some mixes lose their kick after a day or two, especially vitamins, peroxide-based cleaners, or some plant foods. Cool, dry, airtight containers stretch shelf life. Old habits, like leaving lids off or scooping out with wet spoons, spell trouble. Mold and clumping signal you’ve gone too far. Clear labeling with date and strength saves you from future confusion, keeping routines smooth and safe.
If a product refuses to dissolve, patience and technique go a long way. Try warming the liquid, switch your mixing tool, or break lumps before adding. Sometimes gentle crushing with a mortar and pestle makes fast progress. For everyday powders that keep forming stubborn blobs, switch to smaller, repeated stirrings and sprinkle gently. If sediment remains, it sometimes means you’ve overloaded the water—for these cases, you’re better off splitting the dose across several smaller volumes.
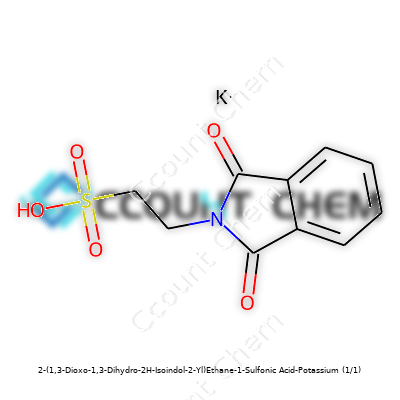
| Names | |
| Preferred IUPAC name | Potassium 2-(1,3-dioxoisoindolin-2-yl)ethane-1-sulfonate |
| Other names |
ACES potassium salt ACES K salt ACES buffer potassium salt N-(2-Acetamido)-2-aminoethanesulfonic acid potassium salt N-(2-Acetamido)-2-iminodiacetic acid potassium salt |
| Pronunciation | /tuː ˈwʌn θri daɪˈɒksoʊ ˈwʌn θri daɪˈhaɪdroʊ tuː eɪtʃ aɪˈsɔɪnˌdoʊl tuː aɪl ˈɛθeɪn wʌn sʌlˈfɒnɪk ˈæsɪd pəˈtæsiəm wʌn əv wʌn/ |
| Identifiers | |
| CAS Number | 132186-41-3 |
| 3D model (JSmol) | `4ZV9` |
| Beilstein Reference | 149276 |
| ChEBI | CHEBI:132805 |
| ChEMBL | CHEMBL3622972 |
| ChemSpider | 22670195 |
| DrugBank | DB08647 |
| ECHA InfoCard | 03b48bec-bb8b-403a-81c4-ffb6ffed7b4e |
| EC Number | 25151-86-4 |
| Gmelin Reference | 115284 |
| KEGG | C03601 |
| MeSH | D013099 |
| PubChem CID | 15126302 |
| RTECS number | GV8950000 |
| UNII | A9H2V4283D |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C10H8KNO5S |
| Molar mass | 361.42 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.69 g/cm³ |
| Solubility in water | soluble |
| log P | -2.5 |
| Acidity (pKa) | -2.1 |
| Basicity (pKb) | 7.61 |
| Magnetic susceptibility (χ) | -62.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.610 |
| Dipole moment | 6.7492 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 273.2 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1101.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1423.4 kJ/mol |
| Hazards | |
| Main hazards | H315; H319; H335 |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 220.7 °C |
| LD50 (median dose) | LD50 (median dose): > 2,000 mg/kg (Rat) |
| NIOSH | TT4925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
Phthalimide Ethanedisulfonic acid Potassium phthalimide N-(2-Sulfoethyl)phthalimide Phthalic anhydride Isoindoline-1,3-dione |