Scientists have always valued curiosity. The history of 1H,5H-Pyrazolo(1,2-A)Pyrazol-1-One, 2,3-diamino-6,7-dihydro-, dimethanesulfonate starts with that spirit. In the late twentieth century, researchers wanted new building blocks for pharmaceuticals and fine chemicals. Synthetic organic chemists began tinkering with strategies to couple pyrazole rings, not just for the thrill of making something new, but for the urge to solve medical puzzles. During those years, experiments using pyrazolone skeletons and selective sulfonation unlocked a versatile toolkit. The shift to dimethanesulfonate salt came from researchers wanting better solubility profiles and safer handling. Funding often went to teams that could take old lab discoveries and give them a new twist fit for high-throughput screening, so this molecule was primed for exploration, not as a finished drug, but as a step that could go in different directions. It grew from a curiosity on bench tops in university labs to a staple found in synthetic routes across research centers, all stemming from the old-fashioned drive to build something a little bit better than what came before.
Step into a lab stocked with chemical intermediates and you will find jars labeled with tongue-twisting pyrazolopyrazole derivatives. This particular compound, with its long name, pops up in catalogs for medicinal chemistry, custom synthesis, and preclinical assays. Chemists like it for the balanced trade-off between reactivity and stability. Companies tend to offer it as a crystalline powder, tightly sealed to keep moisture out. Often catalog numbers skip the headaches of pronunciation. Its appeal lies in how it acts as a platform for both academic study and commercial application — not as glamorous as some blockbusters, but always the quiet workhorse making more elaborate science possible.
Digging into its actual character, the dimethanesulfonate salt brings clear benefits for storage and use. As a white or off-white solid, the powder is easy to weigh, doesn’t clump too badly in dry cabinets, and dissolves smoothly in polar solvents. The diamino groups attached to the backbone serve as handy points for derivatization. Surface-level looks never tell the whole story, so it takes measured melting points and solubility data to truly define a compound’s reliability. Labs measuring batch quality find this salt less prone to hydrolysis than related pyrazoles — a boring detail until comfort with handling comes into play. From a chemical point of view, the electron-rich sites across the rings attract attention for further modification, making it valuable for anyone chasing tailor-made molecules.
Researchers face no shortage of strict documentation. This compound is no exception. Technical sheets routinely state purity (usually surpassing 98%), moisture content, and trace contaminants down to parts per million. Those prepping for quality audits see that each bottle carries information on synthesis date, retest timelines, recommended storage, and batch traceability. Many producers print hazard codes based on GHS standards, and laboratories demand safety data sheets before a shipment gets anywhere near the bench. Industry practice requires child-resistant containers and tamper evidence where controlled substances regulations touch research. As study projects move from exploratory runs into regulated work, analysts comb over every specification to prove integrity in reporting and results.
Synthetic routes for this molecule have matured through trial, failure, and tweaks borrowed from unrelated reactions. One approach sees the stepwise condensation of hydrazine with a dicarbonyl precursor, tailoring the reaction pH to avoid byproducts. The raw product needs to be purified — often recrystallized out of a mixture like ethanol-water to remove colored side products. After that, the conversion to the dimethanesulfonate involves treatment with methanesulfonic acid under controlled temperature, taking care to quench excess reagent and remove volatile residues through reduced-pressure drying. Technicians recognize the rhythm of careful addition and patient waiting for crystals to form, followed by repeated rinsing to reach high purity. Each batch carries the memory of that process, stamped in lot records and trusted by those who repeat the synthesis.
The dual amino groups turn this molecule from a static compound into a launching pad for more complex chemistry. Under mild conditions, chemists can form Schiff bases, couple to acyl reagents, or use diazotization as a door to aromatic substitution patterns. The heterocyclic framework stands up to strong nucleophiles, allowing for functionalizations without ring opening. In the hands of skilled researchers, simple modifications turn the starting material into enzyme inhibitors, binding probes, or ligands for metal complexes. Academics push to find greener reaction conditions — less solvent waste, lower energy input — while patent filers map each transformation hoping to stake a claim on the next useful derivative for commercial exploitation. These laboratory explorations echo back through generations of chemists who understood that versatility is the core trait valued in any intermediate.
Product naming stays messy, as language evolves faster than lab conventions. Chemists use systematic names describing each functional group, but catalogs often abbreviate to forms like Pyrazolopyrazolone dimesylate or just call it by a research code set by the supplier. Journals and patent filings might use IUPAC styling, marking up each nitrogen and carbon, whereas procurement agents stick labels supporting faster searches through inventory systems. Researchers trading samples or talking at conferences whip out the most convenient shorthand, making translation between lab notebooks and invoices a regular challenge. Naming quirks never go away, but those handling this compound learn to spot the tell-tale patterns in both the technical and the commercial sphere.
Handling this compound means more than just following a checklist. People who work long hours with chemical intermediates know that gloves and goggles shield from splash risks, but real safety comes from experience and habit. Methanesulfonate salts don’t pose some of the extreme hazards found in older reagents, but powder dust means respiratory protection gets a spot in lab SOPs. Hoods run nonstop during weighing and transfer. Disposal follows local environmental rules, and any container hitting the waste bin carries a chemical log for down-the-line traceability. Companies with big health and safety teams run annual refreshers, and researchers share stories of small mistakes from the field — all reinforcing the lesson that health counts more than deadlines.
Applications branch out far beyond what the first inventors imagined. Pharmaceutical groups use the molecule as a precursor for kinase inhibitors or antiviral agents, leveraging those amino groups for rapid construction of candidate libraries. Material scientists test it in dye chemistry, probing stable electronic properties. Some agricultural chemists sniff around pyrazole derivatives for pest management trials. Its role as a research tool carries real weight in academic circles, where ease of derivatization helps prove or disprove biochemical mechanisms. Every year, technical reports shed light on a new pathway borrowed from this scaffold, proof that no single field can claim exclusive rights to such a flexible chemical.
Innovation comes from repetition and creative mixing of old and new. University teams keep publishing fresh routes to make purer, faster, or cheaper variants of this salt. Analytical groups focus on better chromatographic separation, aiming for less background interference in downstream work. Pharmaceutical companies, pressed by cost constraints, invest in continuous flow reactors to raise throughput, testing greener solvents in the same breath. Custom synthesis shops work with automation and robotics, reducing human error and bringing turnaround times under tight control. R&D managers watch regulatory trends on accepted reagents, scanning for any sign their favorite intermediate may wind up restricted. Progress happens both through big breakthroughs and slow, persistent improvement, bolstered by the community’s willingness to share hard-won experience.
The question of safety does not get shrugged off. Preclinical groups collect data on acute and chronic exposure, sometimes using in vitro screens, sometimes employing small animal models. Publications track patterns in enzyme inhibition, mutagenicity, and off-target reactivity. Methanesulfonate salts generally earn a milder toxicity profile than other strong acids or alkylating agents, but caution stays in place for novel modifications. Standard reference labs run battery tests for skin and eye irritation, with findings summarized in safety data sheets. Toxicologists keep asking for clearer mechanistic understanding, knowing full well that surprises lurk in every untested substrate or unexpected metabolite. For all its utility, nobody assumes that widespread adoption means complete safety, so continuous vigilance remains the rule.
Looking ahead, prospects appear promising for anyone invested in chemical innovation. As the pharmaceutical industry chases new therapies, demand rises for reliable intermediates that allow fast pivots between candidate compounds. Eco-friendly synthesis methods stand to gain traction, especially with pressure from regulators and sustainability initiatives. Chemoinformatics and AI-driven retrosynthetic planning keep identifying derivative possibilities branching from this scaffold. I see more collaborations between academia and industry, each side eager to push boundaries on efficiency, biological relevance, and lower environmental impact. The world of specialty chemicals keeps churning out fresh applications — and in that churn, this compound finds new relevance year after year, speaking to the stubborn creativity of people who refuse to settle for the status quo.
If you spend some time around laboratory benches or thumb through pharmaceutical research, you might stumble across names that look more like tongue twisters than chemicals. 1H,5H-Pyrazolo(1,2-A)Pyrazol-1-One, 2,3-Diamino-6,7-Dihydro-, Dimethanesulfonate counts as one of them. Yet behind these complicated names, you’ll usually find molecules quietly fueling big moments in medicine and industry. This one has built a reputation among chemists, mostly because of what the two-diamino-pyrazolopyrazolone structure brings to the table in terms of biological activity and versatility.
Most researchers learn early about heterocyclic chemistry. Pyrazolopyrazolones belong to a tight-knit family of ‘privileged scaffolds’ that help scientists develop medications or specialty catalysts. In this case, that long chemical name stands for a scaffold with pockets ready to tinker with enzymes or block out problematic biological switches. The dimethanesulfonate salt version offers better solubility — handy for drug-making or for chemical reactions that depend on everything dissolving nicely in a flask.
This compound has no record as an official approved drug, yet its structure offers leads in antimicrobial, anti-inflammatory, and antitumor research. I’ve seen labs turn to it as a base for building experimental drugs targeting aggressive cancer or as a molecular probe for trickier targets in cell biology. Some studies suggest these kinds of pyrazolopyrazolone backbones can home in on proteins central to diseases hard to treat. In early-stage research, derivatives have slowed cancer cell growth in petri dishes or helped chemists develop new enzyme inhibitors, which matters when options for certain rare diseases run low.
Beyond the medicine cabinet, synthetic chemists use it as a starter block for building new compounds. Materials science teams like playing with the way it joins with other molecules, sometimes creating new dyes, stains, or polymers. Pyrazolopyrazolone parts can anchor onto metals or slip into new sensor materials, helping to catch traces of toxins or other hazards in the environment. If you work in chemical manufacturing or specialty coatings, compounds like this might sneak into catalyst development stretches or show up in tests for new binding agents.
Every promising molecule brings challenges. Data on the environmental impact and safety profile for this salt form run pretty thin. Regulatory bodies and research partners will need to watch out for toxicity, side effects, and how materials based on this scaffolding behave in real-world settings — whether they end up in rivers, soil, or recycled products. Fact-checking and transparency matter, so following guidelines and staying honest about what’s known and what’s guesswork count for a lot. Chemistry brings excitement, but safety nets can’t get skipped.
For those looking to make a mark with new medicines or eco-friendlier chemical processes, this compound tells a familiar story: opportunity tied up with risk and responsibility. Building out reliable toxicity data, improving waste protocols at the bench, and pushing for independent testing will shape its future uses. Open sharing across industry, academic circles, and regulators builds a healthy knowledge base — because the best breakthroughs come when smart minds share what works and what doesn’t. Steady research, fact-driven dialogue, and a willingness to ask tough questions will take this molecule — and others like it — to more meaningful places.
Every chemical compound tells its own story inside a container, and proper storage writes the ending. I’ve seen folks treat chemicals like dusty books—shelved and forgotten, nothing else. Truth is, the bottle’s label only starts the conversation about safety and utility. It’s easy to stack containers in a corner, but without care, even the calmest powder can cause a commotion.
Temperature swings test the patience of almost every compound. For instance, unstable ones can clump up, degrade, or even release fumes, especially in rooms where the thermometer does gymnastics. I remember walking into a garage lab in July; half the glassware fogged up, and one compound had changed color. That’s chemistry going sideways. Even stabilized compounds have limits. A quick look through material safety data sheets often recommends cool, dry, and well-ventilated spaces for a reason—reactivity tends to go up with heat, and humidity acts like a hidden switch.
Humidity finds every crack, every loose cap. Powders love to soak up moisture until they clump and lose potency. It’s like leaving salt on a picnic table overnight—next day it’s a brick. Some compounds, like certain pharmaceuticals and fine chemicals, act the same way. You want tight seals and maybe even desiccants. I’ve worked with folks who use silica gel packets inside cabinets. Not glamorous, but absolutely effective.
Light changes more than mood in a workspace. Sunlight nudges molecules into new shapes—sometimes harmless, sometimes completely useless. I had a colleague lose an entire batch of photo-sensitive dye because someone left the storage room window open on a sunny morning. Brown bottles and opaque containers don’t just look good; they mean the difference between reliable results and a ruined experiment.
Air exposure ranks up there with moisture as a spoiler. Oxygen does more than rust metal; it can make a perfectly stable powder turn rancid or encourage unwanted chemical shifts. The sharp smell at the top of an opened bottle sometimes gives it away. I always tell rookies: If the cap is loose, it’s just an invitation for oxygen to join the party. Using nitrogen to flush volatile compounds really isn’t overkill where sensitive substances are concerned.
Mix-ups start with messy shelves and missing labels. More than once, I’ve seen the fallout from someone grabbing the wrong bottle in a rush. I use labels with bold letters and log every addition. It takes minutes and saves months of headaches. Separate acids and bases, flammables from oxidizers. The color-coding system isn’t just for show. Those lines on the shelves matter once something spills or leaks.
Secure storage calls for more than one-size-fits-all rules. The best labs and workplaces I’ve set foot in prioritize clear information—up-to-date safety data sheets within arm’s reach, clear instructions on every shelf, and a simple checklist taped to the door. Spill containment trays, local ventilation, and double-checks on expiry dates make a difference. It’s never about paranoia; it’s about everyday respect for the quiet power in each container.
In the end, careful storage means fewer mistakes, better results, and, hopefully, a smoother day for everyone working in that space.
Products used in workplaces, garages, and even homes often sneak in danger under the labels. Folks might see bright colors or fancy packaging, but every chemical or industrial item carries risks. Take solvents, for example—these can cause trouble fast. Fumes spark headaches or dizziness. Skin contact sometimes leads to chemical burns, itching, or allergic rashes. Even something as common as bleach can surprise you with how it interacts with other cleaners, pumping harmful gases into the air.
Several years ago, I worked in a busy auto shop. One of the guys mixed brake cleaner and degreaser without thinking much. The fumes sent two of us outside gasping for air with watery eyes. The lesson stuck because hazards rarely announce themselves. It’s not about alarmism—it’s about knowing what you’re up against so you can keep your lungs, skin, and even long-term health intact.
Some products burn easily. Others explode if stored close to a heat source or knocked over onto a running tool. Products like strong acids or lye eat through clothing, shoes, or even concrete floors if spilled. Sometimes you can’t even smell trouble—carbon monoxide sneaks up without warning, and a simple slip in focus may end in the emergency room. More than one recycler I’ve met has handled “empty” drums that still had traces of volatile chemicals. Static electricity, a dropped tool, and the next thing you know, there’s a flash fire.
I don’t bother with complicated language. Before opening any product, read the label. Look for skulls, flames, or warnings in bold letters. Don’t use products with damaged or faded labels—guessing games cost too much. Safety Data Sheets (SDS) make it easy to see what you’re dealing with, from flammability to corrosiveness and long-term exposure risks.
Good sense goes a long way. Always use gloves, goggles, and the right respirator, not just dust masks. Even tough hands or calloused skin won’t block strong solvents or acids for long. Work in a space with plenty of airflow. A fan or open window reduces fumes, but sometimes you need a full ventilation system. Never store chemicals in food containers or soda bottles. Kids or distracted coworkers often grab the wrong item, leading to toxic exposures or accidental poisonings.
Safety isn’t a solo job. Training should happen often, not just on the first day. Teams that do regular drills and talk about “close calls” build habits that protect everyone. I saw a veteran carpenter stop a rookie before he opened a can of poly near an electric sander. Those short conversations make the difference. In any shop or lab, a culture of looking out for each other brings down injury rates.
Trusting instincts and watching for changes in product appearance or smell gives early warnings. It pays to store chemicals in original containers. Don’t stack incompatible items—bleach and ammonia must stay far apart. Have spill kits ready and teach everyone where they are and how to use them. And remember, respect the product; cutting corners never ends well. Putting safety up front keeps the day from turning sideways and lets everyone go home in one piece.
As a former lab tech and someone who’s worked in multiple sectors—food processing, construction, and now healthcare—I’ve seen what picking the wrong product grade can do. Folks outside those industries might picture “purity” as a concern for scientists in white coats, but it touches all of us. Just pick up any medicine, cleaning solution, or water softener and consider: every one relies on using the right material for the job.
Products aren’t one-size-fits-all. Salt isn’t just “salt,” for instance. There’s table salt, industrial salt, and pharmaceutical salt—and only one belongs on french fries. If a treatment plant uses the wrong chlorine grade, drinking water might end up with nasty byproducts. Hospitals don’t skimp here either. A lower purity batch of medical oxygen can trigger complications.
Certain food additives, like citric acid, come in multiple purities. High-purity grades prevent weird flavors in drinks or jams. Lower grades serve well in detergents, where extra precision matters less. I once saw a food start-up grab the cheapest supplier for a jam recipe. The product literally fizzed in the jar, the result of trace reactions from impurities. That batch never hit shelves.
Government agencies set the bar on grades for good reasons. Take the U.S. Pharmacopeia or Food Chemicals Codex—they list minimum standards to protect the public. Slip below those marks and a company risks recalls, lawsuits, or worse. In 2008, a single contaminated batch of heparin, a common blood thinner, caused dozens of deaths. The root cause: someone used material that didn’t hit the right purity mark.
If you run a small business or are sourcing for a bigger firm, it pays to know what kind of purity you need. I’ve seen companies cut corners on ingredient quality, only to spend months cleaning up messes. On the other side, over-specifying purity means paying more for benefits you don’t really need. There’s always a cost calculation—high-purity titanium costs more than standard. If you only need door hinges, aerospace grade makes no sense.
Transparency plays a big role. Sellers must clearly mark purity stats, and buyers should ask for documentation—the certificate of analysis, for example. In construction, if cement contains more impurities than allowed, buildings can fail. In pharmaceuticals, any ingredient used has to match its “grade,” as listed on meticulous paperwork—federal inspectors drop in to review it, and fines fly for mistakes.
Start by checking industry standards. Talk with peers and lean on quality assurance folks—those are the ones who’ve learned the hard lessons. Console cheap options with a checklist: Will it affect safety? Does the application have tight regulations? If something feels off, don’t risk it.
Quality costs money, but so does fixing disasters. If you’re buying for fun projects at home—a little leeway makes sense. If you’re making food, health, or building products, there’s no shortcut worth taking.
Most folks rarely think twice about tossing old chemicals or expired cleaners in the trash or pouring them down the sink. But I’ve seen what happens when these “out of sight, out of mind” moments pile up. Public drinking water ends up with traces of substances like perfluorooctanoic acid. Local wildlife can show bizarre mutations or die-offs. Every step in the wrong direction leaves a mark—sometimes for generations.
The federal government tracks these issues closely through the Environmental Protection Agency. For instance, routine monitoring shows that some of the most common local pollutants come not just from industry, but from homes and small businesses that cut corners during disposal. If enough people dump solvents, batteries, or pharmaceuticals into household trash, the city landfill becomes a ticking time bomb. Chemical fires, leaching into groundwater, and air contamination don’t need major disasters to show up—they’re often the result of hundreds of tiny mistakes.
Disposal starts with identification. I’ve spent afternoons reading bottle labels and pulling up Safety Data Sheets (SDS) on my phone. These sheets explain not only what risks a chemical poses, but also which special rules apply to its disposal. If a product looks unfamiliar or smells especially harsh, I do not guess—I check. Accuracy beats speed every time, especially when the wrong move can threaten neighbors or first responders.
Most towns hold dedicated hazardous waste collection days. Residents line up in their cars with paint cans, old pesticides, and mystery fluids in the back seat. The line might look long, but trained staff unload the stuff and pack it for safe transport. These drives send hazardous waste either to certified treatment facilities or special landfills with liners that prevent leaks. This approach prevents illegal dumping, reduces risks to waste workers, and cuts off the path to water, soil, or air contamination.
I’ve lived in a building where one neighbor poured motor oil into the gutter. The stench lingered, and the next time it rained, the oil floated down the street toward the sewer. That experience shifted my outlook. It takes only a few minutes to find a drop-off location for used oil or electronics. Many auto shops even accept oil for free, reclaiming it for recycling.
Hospitals and pharmacies now collect old medications, locking them in bins that eventually go to high-temperature incinerators. These practices prevent antibiotics, hormones, and other chemicals from polluting rivers and lakes. It’s a system that works best with strong community participation—people who care enough to ask where that leftover pill bottle belongs.
Small actions make a big difference—a clean garage, organized storage shelves, and a habit of asking questions. These steps lead not just to safer homes, but healthier towns. Proper disposal is a collective effort, rooted in choices made by individuals who understand that waste never truly disappears; it moves, reacts, and sometimes comes back when we least expect it.
Hazardous chemicals demand respect. They bring comfort and convenience but carry risks if ignored. Honest conversations and simple habits help protect children, pets, and the ecosystems we often take for granted. Building these routines now pays dividends for future generations. The right disposal method rests on information, community effort, and a willingness to invest time in doing the right thing, even when no one is watching.
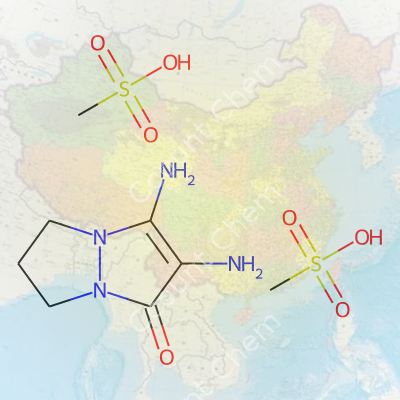
| Names | |
| Preferred IUPAC name | dimethylsulfonato(2,3-diamino-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one) |
| Other names |
3,6,7-Triamino-1,2,4,5-tetrahydro-1,5-dioxopyrazolo[1,2-a]pyrazolium dimethanesulfonate 3,6,7-Triamino-1,2,4,5-tetrahydro-1,5-dioxopyrazolo[1,2-a]pyrazole dimethanesulfonate Triaminopyrazolopyrazolone dimethanesulfonate |
| Pronunciation | /paɪˈræzəloʊ ˈpaɪrəzoʊl wʌn tu θri daɪˈæmɪnoʊ sɪks ˈsɛvən daɪˈhaɪdroʊ daɪˌmɛθeɪnˈsʌlfoʊneɪt/ |
| Identifiers | |
| CAS Number | 28832-72-4 |
| Beilstein Reference | Beilstein Reference: 6062121 |
| ChEBI | CHEBI:131346 |
| ChEMBL | CHEMBL151905 |
| ChemSpider | 22087348 |
| DrugBank | DB12917 |
| ECHA InfoCard | ECHA-InfoCard-100.176.903 |
| EC Number | EC 616-375-1 |
| Gmelin Reference | 58879 |
| KEGG | C18504 |
| MeSH | D013696 |
| PubChem CID | 70966121 |
| RTECS number | WS5690000 |
| UNII | 8ZUP7SKF1L |
| UN number | UN3474 |
| CompTox Dashboard (EPA) | DTXSID0049812 |
| Properties | |
| Chemical formula | C6H12N6O2·2CH4O3S |
| Molar mass | 480.52 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.65 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.9 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.52 |
| Basicity (pKb) | 6.38 |
| Magnetic susceptibility (χ) | -84.8×10^-6 cm^3/mol |
| Refractive index (nD) | 1.670 |
| Dipole moment | 5.6 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.2 J/mol·K |
| Pharmacology | |
| ATC code | N02BB02 |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1-1-0 |
| LD50 (median dose) | LD50 (median dose): 174 mg/kg (intravenous, mouse) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 mg/m3 |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 1H,5H-Pyrazolo(1,2-A)Pyrazol-1-One, 2,3-Diamino-6,7-Dihydro-, Dimethanesulfonate. |
| Related compounds | |
| Related compounds |
Pyrazolopyrazole derivatives 1H-pyrazolo[1,2-a]pyrazole 1H,5H-pyrazolo[1,2-a]pyrazol-1-one Diaminopyrazole compounds Dimethanesulfonate salts Aminopyrazolone derivatives |