Long before 10-camphorsulfonic acid gained attention in modern chemical circles, folks in the pharmaceutical and fine chemical world spent years searching for reliable acid catalysts that could go beyond just sulfuric or hydrochloric acid. As research dug deeper into the properties of camphor—an old standby for medicinal purposes—chemists realized the potential sitting quietly in this unique bicyclic structure. By the late 20th century, new methods for transforming camphor into its sulfonic acid derivative swept across organic chemistry labs, giving researchers a powerful tool with milder properties than more corrosive acids. The steady progress in synthetic techniques kept raising both yield and purity, setting the foundation for industrial-scale manufacturing. Watching these changes firsthand over decades, the evolving routes and applications for 10-camphorsulfonic acid highlight how persistent curiosity leads to practical solutions in chemistry.
10-Camphorsulfonic acid, or CSA as it’s often called, stands out as a solid acid in the toolkit for organic chemists. Chemically derived from natural camphor, this compound holds stable in air and moisture, delivering strong yet manageable acidity. Packed as a white crystalline powder, CSA gets shipped globally as one of the better solid acid catalysts for precise synthetic transformations. Unlike mineral acids, CSA seldom brings about violence in reaction flasks—less hassle, fewer accidents, better control. Once you’ve used it for a few runs, the flexibility it offers becomes hard to live without, especially in settings where product purity cannot take a back seat.
At room temperature, CSA takes the form of white, slightly sticky crystals that melt between 186°C and 190°C—far higher than many organic acids. Its solubility in polar solvents like water, methanol, or acetonitrile paves the way for easy handling and preparation of solutions. With a molecular weight of about 232 g/mol and a pKa near -1.2, the acid strength comes close to those heavy hitters in the mineral category, but with many of the toxicological risks dialed down. This mix of solubility, stability, and strength launches CSA to the front of the line for reactions demanding tight acid catalysis without side products.
Manufacturers label CSA with clarity—batch purity regularly exceeds 99% by HPLC or titration, and any trace impurities (like inorganic salts or residual camphor) get tabs kept on them via detailed COAs. With the UN number 3261 for corrosive solids, safety data find their way onto the packaging, along with recommended storage conditions (cool, dry, away from sunlight). Physical lot details, weight, and purity each find a home on every package label. From decades spent reviewing shipment documents, consistency matters most, and tight labeling standards help those of us in the chemical trades avoid confusion—a win for both safety and accuracy.
Preparation often follows a simple but reliable route: concentrated sulfuric acid brings camphor into submission, replacing a hydrogen on the ring with a sulfonic acid group through electrophilic aromatic substitution. After the reaction, careful work-up—lots of dilution, neutralization, and repeated washing—yields high-purity CSA, removing unreacted camphor and side products. Some processes add extra steps, like crystallization from water or acetone, to hit pharmaceutical-grade purity. Later advances have explored milder sulfonating agents or greener solvents, but the time-tested sulfuric approach still dominates. Those spent years hunting for better yields or easier purifications always come back to the same simple sulfonation, trading off efficiency against environmental concerns.
CSA shines brightest when it kicks off acid-catalyzed transformations. Esterifications, dehydrations, or rearrangements go smoother with this reagent, and as a chiral auxiliary, its camphor skeleton helps drive stereoselectivity. The sulfonic group withstands moderate heat and even oxidation, letting chemists tinker with derivatives—a world of camphorsulfonates springs up from copper, sodium, or lithium salts, each with tweaks to solubility or reactivity. CSA can undergo further modification by halogenation or even amidation, ready for custom-tailored applications. Watching CSA at work in the bench-scale flask or kilo-lab always brings a sense of control and predictability, not always found in traditional acid catalysts.
CSA hardly wears just one name on the shelf. You’ll run across names like 10-Camphorsulfonic acid, (1R)-(-)-10-Camphorsulfonic acid, (-)-CSA, Camphorsulfonic acid monohydrate, and (1R)-10-(Sulfooxy)bornan-2-one in catalogs and regulatory lists. International suppliers assign their own codes—sometimes even the more cryptic "CSA acid" or the chirality-defining prefixes for the optically pure (R)- or (S)- enantiomers. No matter the official title, it’s been my experience in both academic and industrial settings that chemists know exactly what’s meant by CSA; chatter in the lab or at a conference cuts right through branding.
CSA commands respect on the bench. Skin or eye contact brings irritation, sometimes severe if handled without basic gloves and goggles. Inhalation of dust irritates mucous membranes, so fume hoods get regular use during weighing or mixing. Safety Data Sheets spell out the risks—corrosive label, proper storage, and instructions for spill control. Standard procedures focus on dilution first and neutralization later, using soda ash or sodium bicarbonate to tame accidental spills. Long experience dealing with acids teaches caution pays off—keeping CSA containers tightly sealed, cool, and out of sun avoids decomposition and moisture issues. Waste disposal falls under hazardous protocols due to its acidity and sulfonate structure—no drain dumping allowed. Regular refreshers on safety gear and spill plans should run before bringing CSA into a new facility to keep old hands and new hires alike out of trouble.
Chemists in both bench and pilot plants use CSA as an acid catalyst for preparing pharmaceuticals, flavor compounds, and advanced materials. Synthesizing cephalosporins, steroids, or alkaloids gets a boost in both yield and purity thanks to stronger control over reaction conditions. CSA’s sulfonic group helps drive regioselectivity and stereochemistry, opening the door for asymmetric synthesis—something no mineral acid offers so easily. Its mildness lets delicate structures survive, a real bonus once you’ve lost enough batches to stronger acids. Nutritional supplement manufacturers, fragrance designers, and even battery researchers pull CSA off the shelf, seeing its versatility stretch from drug synthesis to advanced battery electrolytes. In my own experience, turning to CSA solved countless headaches in Friedel–Crafts runs, often rescuing both the chemist and the project from harsher alternatives.
CSA sits on the front lines of chiral chemistry research. Scientists keep probing new uses for both the acid and its metal salts, engineering ways to recover and recycle CSA to reduce process costs and environmental load. Process development teams chase greener sulfonation routes—seeking better atom economy, waste mitigation, and recoverable catalysts. Recent R&D headlines focus on asymmetric reactions, aiming to unlock higher enantiomeric excesses in complex syntheses. Collaborations between academic labs and pharma companies keep unearthing new derivatives and applications, some targeting green chemistry awards, others chasing untapped markets. Over the years, seeing CSA-involved patents multiply speaks volumes about its staying power—it’s not a passing fad but a versatile staple.
CSA’s toxicity falls well below that of nitric or sulfuric acids, but nobody in chemical safety treats it casually. Animal trials and cell culture tests show irritation to skin, eyes, and respiratory tracts at moderate exposure, dropping off sharply with proper PPE. Although metabolic breakdown tends to clear CSA from mammals with few lasting effects, chronic exposure gets flagged as a risk, especially for personnel running repetitive batch work. Environmental monitoring surveys have yet to spot substantial groundwater contamination, probably thanks to advisable containment and disposal protocols. The industry keeps an eye on allergenicity and potential byproducts, running regular reviews to stay ahead of emerging risk data. My own run-ins with CSA, like many chemists’, left no permanent marks—gloves and closed-toe shoes work wonders in a world of potent acids.
Looking ahead, CSA’s future stretches beyond classic catalysis. Process-friendly derivatives with higher solubility, lower cost, or even biocompatibility could pave the way for greener synthetic chemistry. Demand from pharmaceuticals, electronics, and clean energy research keeps suppliers tweaking purity and scale-up options. As regulations tighten around chemical waste, CSA’s reusability and lower hazard make it attractive for forward-thinking institutions. Automation and AI in chemical process optimization increasingly flag CSA for roles in complex reaction networks, hinting that its heyday is far from over. After decades of hands-on work with acids both mild and brutal, CSA continues to show up as a reliable, versatile solution with new chapters yet to be written—something few specialty chemicals can claim in today’s rapidly changing labs.
10-Camphorsulfonic acid, often called CSA, finds uses that pop up in both laboratories and industrial workshops. I remember reading an old college lab manual and being struck by how certain white powders influence reactions miles away from consumer view. CSA is definitely one of them. The way it shows up right at the crossroads of synthesis and manufacturing has given it a pretty important role.
Let’s get into why CSA pops up in so many chemical syntheses. In making pharmaceutical intermediates or specialty chemicals, CSA serves as a strong acid catalyst. Its molecular structure means it can speed up reactions like esterifications and acetalizations, making processes run faster without churning out a lot of unwanted byproducts. Chemists lean on CSA because it’s a solid acid that doesn’t bring along excess water the way some liquid acids do. That reliable, dry push gives it a reputation for clean chemistry and predictable results. The fact that CSA has a camphor backbone makes it helpful in guiding reactions toward certain outcomes, a trick most chemists appreciate when product purity really counts.
CSA’s influence goes beyond pure synthesis. In the pharmaceutical world, this acid is often the go-to for forming salts of drug molecules—especially when those molecules start with basic or amine functional groups. By reacting with those amines, CSA transforms tricky-to-handle oils into crystalline, easily purified solids. That simple change unlocks stability that’s needed for drugs heading to the pharmacy shelf. I’ve seen a couple of patent filings where CSA stands out in the salt formation step, helping a tricky molecule get through the regulatory gauntlet by improving its shelf life and solubility.
The camphor ring isn’t just decorative. It brings a “handedness” into the reaction flask. In asymmetric synthesis, CSA gives chemists leverage to tip outcomes one way or the other. Getting the right isomer makes all the difference in farming useful pharmaceuticals. The fact that CSA can steer these reactions is a big deal. Drug makers can minimize the time wasted on separating out mirror-image byproducts. If you know someone researching painkillers, antibiotics, or psychiatric drugs, ask them about CSA. There’s a good chance it helped guide their molecule in the right direction.
CSA turns up in a few other pockets of the world, too. Electrochemists have found it helps in doping polymers. Some paper from a few years back described CSA improving the conductivity in polyaniline, a material used in batteries and sensors. It enables thin films with better conductivity—so that’s a plus for making next-generation electronics just a bit more efficient. The acid also plays a part in producing certain flavors and fragrances, where its strong acidic power helps shape key intermediate compounds with a reliable, safe workflow.
No compound offers a free ride. Anyone using CSA regularly has to handle it carefully. It’s got a strong odor, it’s corrosive, and direct contact with skin or eyes spells trouble. Safety protocols go from theory to daily practice any time CSA gets handled. The ongoing shift toward green chemistry means more eyes are now on how to recycle or replace CSA without losing performance. Some chemists look for milder alternatives where possible, but CSA’s reliability and versatility keep it in the game. Those who work with it understand its quirks, respect its risks, and recognize its many contributions. That’s how a small molecule gets a lasting place in science and technology.
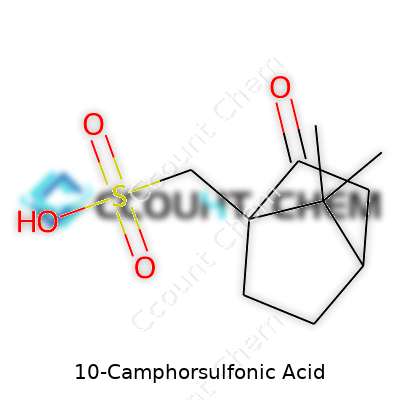
10-Camphorsulfonic acid carries the chemical formula C10H16O4S. This formula defines a sulfonic acid derivative made from camphor, a bicyclic monoterpene. You see the backbone of camphor along with the powerful presence of a sulfonic acid group. That extra group changes everything—it gives the compound a whole different set of behaviors and advantages, especially in the worlds of organic synthesis and pharmaceuticals.
This compound comes up a lot in labs. For anyone who’s worked with organic reactions, 10-camphorsulfonic acid becomes a familiar tool. Chemists reach for it because it’s strong enough to protonate a wide range of substances. I remember prepping some asymmetric syntheses, where getting reaction conditions right was crucial. Regular acids fell short, but this one delivered consistency and control. That level of reliability isn’t common, and you start to notice the difference at the bench when reactions don’t fizzle or go sideways because of inconsistent acid strength.
C10H16O4S isn’t just a string of characters. The arrangement speaks to its function. The camphor structure brings rigidity to the molecule. The sulfonic acid group adds the big punch of acidity. Add both together and you’ve got a solid acid catalyst that works cleanly in organic solvents. In practical terms, folks in medicinal chemistry and fine chemical manufacture lean on this compound to facilitate clean product isolation—fewer byproducts, easier purification, better yields. These matters grow more important the more frequently you run reactions at a manufacturing scale or when you want to avoid potential contamination in a pharmaceutical batch.
10-Camphorsulfonic acid plays a recognized role across organic and medicinal laboratories precisely because its formula C10H16O4S translates to robust acidity (pKa around -1.2 in water). Data from peer-reviewed journals and chemical suppliers highlight how this compound stands out when working in both academic and industrial research settings. On the flip side, its strong acidity means real care needs to enter storage and disposal practices. Direct skin contact stings, and inhaling any dust or fumes can irritate airways. Proper gloves and a fume hood matter each time—no exceptions.
There’s an ongoing push in chemistry to refine tools for selectivity and waste reduction. While 10-Camphorsulfonic acid already enables efficient reactions, researchers have started exploring greener synthesis pathways and recycling strategies for sulfonic acids. The hope is to cut down on chemical waste, trim costs and improve safety for folks working every day in the lab. More sustainable reactions don’t just help the planet—they also mean lower overhead for small labs that might not have access to expensive purification equipment. The formula C10H16O4S might look simple, but its impact stretches far beyond the letters and numbers.
10-Camphorsulfonic acid doesn’t come up in daily conversation, but it plays a key role in chemistry labs and manufacturing. You see its name pop up in pharmaceutical settings and labs dealing with organic synthesis. The way it dissolves—or doesn’t dissolve—in water can either help reactions take off or bring things grinding to a halt. It’s more than a trivial question for researchers, especially when you’re planning for purity, safety, or simply aiming for a product that actually works as expected.
Before reaching for this reagent, people often ask if 10-camphorsulfonic acid actually mixes with water. According to trusted chemical handbooks and the experience of researchers, it dissolves in water quite well. This isn’t just a textbook fact; when I’ve weighed out this stuff in the lab, spilled grains vanished with a few squirts of distilled water. For those of us working with limited lab budgets, this means you won’t need expensive or hazardous solvents just to get this acid into solution. You only reach for water, which is about as handy as it gets.
Why does this happen? The big sulfonic acid group clings tightly to water molecules, forming hydrogen bonds. You end up with a clear, reliable solution instead of a cloudy mess. That lets you move forward with reactions in fields such as chiral resolution, where water-soluble acids play a crucial part.
Chemists need practical info, not just theory. Solubility in water means less hassle with clean-up, and easier measurements. You rinse glassware with water and it actually gets clean, no residue left behind. It’s a straightforward solution—you’re not reaching for a jumble of hazardous waste containers every time.
This solubility opens the door for greener chemistry. Using water as a solvent means fewer problems with disposal and worker exposure. You don’t end up burdening local water systems with persistent organic pollutants. Plus, water costs far less and doesn’t leave a heavy environmental footprint, so production lines get to save money and stick closer to environmental targets.
Easy water solubility has a downside: you must store 10-camphorsulfonic acid correctly to stop accidental clumping from humidity. Once, a new technician at our lab left the acid jar open. Moisture in the air turned the crystals into a sticky, partly dissolved mass. Good storage practices—airtight containers and low-humidity rooms—solve the problem. Overlook that, and you’re left chipping away at a lump instead of spooning easy-to-manage powder. Attention to detail stops headaches down the road.
Knowing that this acid mixes with water provides more than technical comfort; it means safety crews can draft realistic spill response plans, and educators can give students practical demonstrations without health concerns. Reliable data matter—too many times, misleading product specs leave even seasoned folks guessing. Support from reputable material safety data sheets and decades of hands-on work form the backbone here, giving chemists and industry clear guardrails to operate within.
Instead of questioning each batch, you can go straight to planning, mixing, and learning—without unexpected surprises, extra costs, or emergency workarounds. That’s how reliable, practical science helps good ideas become real-world solutions.
10-Camphorsulfonic acid stands out in the world of chemistry. Its unique structure brings real value to a lab, whether someone is working on pharmaceuticals or experimenting with organic synthesis. I remember the first time I handled this crystalline powder. There was excitement about its potential and wariness about its strong, pungent smell and acidity. If that jar finds itself on a cluttered bench, all sorts of things can go wrong. Lab safety becomes more than a checklist—it's about respecting chemicals like this for what they can do and what they can cause.
Water in the air loves to sneak into open containers. 10-Camphorsulfonic acid loves water even more. It pulls moisture from the air, clumping, degrading, or sometimes liquefying entirely. Anyone who has dealt with caked chemical powders understands the stubbornness: weighing becomes tricky, measuring turns imprecise, and soon the original material can't be trusted at all. Lab staff have told me stories about ruined batches that forced them to start over, costing time and resources. Dry, tightly sealed containers change that game. Desiccators with silica gel or other drying agents offer an extra layer of protection, especially in damp climates. This simple routine—returning the jar to its dry storage spot after each use—makes a real difference over the years.
Many forget the hidden damage that sunlight brings to sensitive reagents. UV rays and heat spark unexpected reactions, fade labels, and speed up decomposition. 10-Camphorsulfonic acid doesn’t put on a dramatic show when exposed, but its quality drops. In lab courses, storing acid powders in dark glass or keeping them in cabinets away from direct sun helps avoid those problems. After running pilot projects in bright workspaces, I've learned that even small amounts of regular sun change how chemicals behave. Poor storage here risks more than product loss—it sets up entire experiments to fail.
Labels do more than keep regulators happy. Swapping caps or confusing containers creates real dangers in fast-paced settings. Clear labeling—including opening dates and hazard symbols—makes shared spaces safer. Small details, like using a permanent marker for date and initials, or sticking to a logbook routine, build better habits. Years of lab work have taught me that labels (including warning stickers) often serve as the last line of defense when fatigue or distraction creeps in.
Strong acids, even organic ones like 10-Camphorsulfonic acid, don't mix well with casual storage. Placing acids far from bases, oxidizers, and incompatible solvents prevents dangerous reactions. A colleague once stored a bottle of perchloric acid near an organic acid, and a minor spill led to a smoky, hazardous mess. Separate, dedicated cabinets—preferably corrosion-resistant—keep the risk low. Locks add another level by keeping unauthorized hands away, and written protocols help new students or staff make smart choices.
Safe, thoughtful storage isn’t just tradition—it’s about protecting people, experiments, and investments. Understanding humidity, sunlight, labeling, and separation comes from experience and attention in the lab. Simple routines can prevent years of trouble and make science move forward more smoothly.
I remember the first time I opened a container of 10-camphorsulfonic acid in a crowded undergraduate lab. The sharp smell caught me off guard, and my gloved hands trembled a bit—especially after my supervisor quietly pointed out that strong acids won’t show mercy to nerves or skin. If you work with this compound, the hazards feel personal. Strong organic acids prove tricky, and camphorsulfonic acid stands in that category.
Direct contact with 10-camphorsulfonic acid leaves a quick sting on the skin or worse, an ugly burn. I’ve seen coworkers scramble for the eyewash station because a single stray droplet slipped past a pair of glasses. Safety goggles aren’t an optional accessory—wrap-around models offer the most comfort and coverage. Long-sleeve lab coats give another important barrier, especially since splashes happen fast and never wait for anyone to grab extra protection. Choose gloves with a strong reputation against acids—nitrile or neoprene works best. Latex fails under acid stress and can actually deteriorate, putting hands at even greater risk.
Inhalation risk runs higher than many expect. Fine dust or fumes from this acid turn the air around your bench into an irritant. Anyone who’s inhaled even small amounts will remember the scratchy throat and rapid cough. Always work inside a chemical fume hood. Critically, fume hoods also protect others in your workspace from surprise smells or accidental aerosolized acid. Never skip this step, even for “quick” procedures. The few extra minutes could save hours at the doctor’s office later.
A solid acid at room temperature, 10-camphorsulfonic acid usually stays stable if left alone. Yet moisture starts a slow breakdown, and high heat amplifies risk. Seal containers tightly after every use and keep them far from sinks or areas likely to get wet. Flammable materials must stay out of the same cabinet to avoid reactions. Label containers in bold ink and check inventory dates regularly. In my lab, once a quarter, we always open every chemical cabinet to double-check seals and look for crystal growth on the outside of forgotten vials.
Spills demand immediate action. Specialized spill kits make a difference—neutralizing powders slow down the spread while acid-resistant scoops and pads handle cleanup. Never reach for paper towels or bare hands. If a spill happens on clothing or skin, a fast rinse with water—at least 15 minutes—beats any hesitation or embarrassment. After a cleanup, collect waste in specially marked containers. Hazardous waste teams at most organizations take over from there, but they only work with well-labeled, sealed material.
Anyone handling 10-camphorsulfonic acid belongs in yearly safety training. In my years of teaching new students, confidence grows with hands-on demonstrations, not just written instructions. Experienced colleagues set examples, reminding everyone to treat every acid bottle like it’s full to the brim and waiting to spill. Talking through near-misses and minor mistakes in lab meetings builds a safety culture where people fix trouble before it grows.
Working with strong acids brings responsibility that becomes clearer with each shift at the bench. Risks never disappear, but habits and teamwork can keep the worst off the day's agenda.
| Names | |
| Preferred IUPAC name | (1R,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-yl hydrogen sulfonate |
| Other names |
(+)-10-Camphorsulfonic acid CSA Camphor-10-sulfonic acid 10-Camphorsulphonic acid Camphorsulfonic acid |
| Pronunciation | /ˈkæm.fər.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 5872-08-2 |
| Beilstein Reference | 1721480 |
| ChEBI | CHEBI:35619 |
| ChEMBL | CHEBI:2787 |
| ChemSpider | 6019 |
| DrugBank | DB11231 |
| ECHA InfoCard | EC 201-995-5 |
| EC Number | 209-190-5 |
| Gmelin Reference | 85982 |
| KEGG | C06811 |
| MeSH | D002156 |
| PubChem CID | 23445 |
| RTECS number | GD5950000 |
| UNII | Y14A2U1Y67 |
| UN number | UN2581 |
| CompTox Dashboard (EPA) | DTXSID7042785 |
| Properties | |
| Chemical formula | C10H16O4S |
| Molar mass | 232.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.346 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Acidity (pKa) | -1.2 |
| Basicity (pKb) | -5.0 |
| Refractive index (nD) | 1.558 |
| Viscosity | 400 mPa·s (20 °C) |
| Dipole moment | 4.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 260 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1635 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye damage. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H318, H335 |
| Precautionary statements | Precautionary statements: "P261, P264, P271, P301+P330+P331, P304+P340, P305+P351+P338, P311, P405, P501 |
| Flash point | 94 °C |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1870 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 10-Camphorsulfonic Acid: "2500 mg/kg (rat, oral) |
| NIOSH | GR2375000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Camphorsulfonyl chloride Camphor Sulfanilic acid |