Chemistry advances in waves, and each new compound often answers an old problem or unlocks a road leading to fresh innovations. The tale of 1-Propanesulfonic Acid, 3-(Cyclohexylamino)-2-Hydroxy-, Monosodium Salt fits this pattern. Interest in sulfonic acids, and derivatives tying together hydrophilic sulfonate groups with hydrophobic rings or amines, grew as researchers looked for stable yet flexible intermediates. Sometime after surging research in surfactants and pharmaceutical intermediates, synthesis of compounds combining cyclohexylamino groups with sulfonic acid emerged. Laboratory chemists explored modifications to boost solubility, control reactivity, and adapt to changing processing needs. The monosodium salt format responded to calls for stable storage and manageable handling. By the late 20th century, reagent suppliers offered this salt consistently to both academic and industrial labs.
People working in chemical analysis, organic synthesis, and biochemistry often scan catalogues for reagents that bridge water solubility with solid-state persistence. This monosodium salt checks those boxes. It brings together the rigidity and bulk of a cyclohexyl group with the reactivity of a hydroxy and an aminosulfonic acid. The combination delivers a molecule that doesn't just dissolve well in water; it stays chemically stable under typical lab storage conditions. Product listings highlight predictable purity levels, batch reproducibility, and a format that weighs and mixes smoothly. Accessibility, safety (relative to free acids), and regulatory labeling appeal to institutional buyers and research planners.
A white or nearly white crystalline powder sums up its usual appearance. Many users appreciate a melting point that supports easy separation from byproducts during purification. Water solubility runs high, owing to the sodium sulfonate group. The molecular weight lands just above 275 g/mol (depending on exact hydration state), which matters in calculation-heavy protocols. Hydroxy and amino groups ensure reactivity with acylating and oxidizing agents, but not so much as to make accidental decomposition a hazard. It plays well with common analysis tools: it’s UV transparent in most assay ranges, and NMR spectra come out crisp with little overlap. Bulk density, hygroscopicity, and resistance to hydrolysis shape shipping and storage policies.
Buyers check for technical specification documents covering purity—often exceeding 98 percent—with clarity on any hydrate or residual solvent content. Labeling focuses on CAS numbers, formula, batch code, date of manufacture, recommended storage temperature, and any hazard statements required under GHS regulation. Package size accuracy comes up often in feedback, as research budgets tighten; suppliers that under-fill or mislabel units lose trust quickly. In lab settings, hazard communication, expiry dates, and supplier lot traceability play just as crucial a role as chemical specs.
I recall a postgraduate lab where brevity worked against us: rushing this compound’s preparation usually led to impure results rather than high yields. Step one starts with reacting cyclohexylamine with 3-chloro-2-hydroxy-1-propanesulfonic acid under mild base, controlling pH to minimize unwanted polymerization. The sodium salt results from post-reaction neutralization, often with sodium hydroxide. Filtration, recrystallization, and careful drying close out the steps. Achieving high batch-to-batch reproducibility calls for precise temperature and pH logs, never just ‘by eye’ mixing. Scale-ups at pilot level need agitation and heat transfer controls, not just direct-mix lab glassware.
Several labs use this compound directly, counting on its hydroxy and amine groups to offer handles for further transformation. Acylation stands out—many users react the free amine with acid chlorides, forming amides that behave differently in biological tests. The hydroxyl can take up phosphorylation or glycosylation for applications in biochemical labeling or to modify polarity. Because the sulfonic acid portion carries a sodium counterion, swaps with other metals or organic cations change properties, sometimes giving access to catalysts with different mechanisms or salt forms for improved crystallinity.
Synonyms can confuse even old pros in reagent selection. This salt sometimes shows up as “CHAPSNa,” a blend from its full IUPAC name, or simply as “Cyclohexylamino-propanesulfonic acid sodium salt.” Some catalogs list the neutral form as “CAPSO sodium salt.” Smart procurement teams cross-reference CAS numbers, not just names, as global markets might favor slightly different naming traditions. For traceability, clear documentation of synonyms helps avoid costly misorders, particularly with specialty chemicals subject to trade or transit restrictions.
Handling requires common sense joined to modern regulatory standards. Safety data sheets classify it as low risk: oral and inhalation toxicity run low, and skin irritation rarely exceeds that of regular mild laboratory bases. At my previous employer, students sometimes mistook white schedule labels for innocuousness—spills around balances still called for immediate clean-up and reporting. Lab managers prefer suppliers that include up-to-date SDSs, easy-to-read GHS pictograms, and compliance documentation matching both local environmental rules and ISO quality certification. Keeping stock away from oxidizers and acids, dry and below 25°C, limits both waste and speaking-to’s from safety auditors.
Usage spreads across life science research, diagnostics, and analytical chemistry. The buffer properties attract biochemists looking for steady pH control, especially for protein purification and enzyme kinetics, where small shifts in acid/base conditions throw whole runs off course. Its solubility profile lets technicians avoid precipitation headaches. Enzyme assays, electrophoresis, and cell lysis protocols benefit from minimal interference. Some industrial testing labs harness it as a background electrolyte in capillary electrophoresis, relying on its zwitterionic balance and lack of UV absorption in critical ranges.
Research teams often tweak compounds like this one to target precise attributes—better stability, softer dissolution, or specialized chelation behaviors. Expanded R&D programs look at alternative counterions or subtle modifications on the cyclohexyl ring for tuned hydrophobicity, intending to suit niche applications. Some drug discovery labs pick up derivatives as buffer agents for high-throughput screening, especially given resistance to microbial growth and low background reactivity. Academic projects use isotopically labeled forms or deuterated variants for advanced spectrometry or metabolic tracking.
Long-run safety studies cover more than touch-and-go handling. Toxicity research, both academic and regulatory, speaks to repeat exposure over days or weeks. To date, acute toxicity shows little cause for worry—oral doses at standard buffer concentrations don’t trigger obvious effects in rodent models. Chronic dosing or inhalation studies appear in isolated literature, usually with results matching or outperforming basic sodium sulfonates for benign impact. Nevertheless, full environmental behavior—especially breakdown products and persistence—draws attention from regulatory panels tracking water discharge and bioaccumulation.
Expect more interest as cleaner analytical methods and tighter bio-process controls grow in demand. Specialty manufacturers explore greener synthesis, aiming to cut down on hazardous intermediates or high-waste side streams typical in traditional acid-to-salt transformations. Automated ingredient tracking and digital labeling, now common in GMP production environments, could push for QR-coded, batch-linked hazard and usage data. Upcoming research sees value in more customized side-chain modifications to suit emerging life science, food safety, and even semiconductor processing markets. As chemical analysis and biotechnology intertwine more tightly, demand stays robust for high-purity, streamlined, well-documented reagents with reliable origin and compositional traceability. The pace of regulatory scrutiny may keep climbing, so transparent documentation and iterative product stewardship top the list of expectations from researchers, technicians, and procurement teams alike.
Life in a chemistry lab never sits still. People might not chat about this compound over coffee, but anyone who’s run complex analysis will recognize the role of 1-Propanesulfonic acid, 3-(cyclohexylamino)-2-hydroxy-, monosodium salt. Most folks call it CHAPS. This zwitterionic detergent quietly supports major breakthroughs in proteomics and molecular biology.
Any time someone picks up a pipette to extract or analyze proteins, they have tough choices. You don’t want delicate proteins clumping together or falling apart before you can study them. Standard detergents break up cell membranes, but older surfactants like SDS or Triton X-100 can wreck protein structure. CHAPS keeps proteins stable. Its unique hybrid structure – part sulfonic acid, part cyclohexyl ring – lets it break apart cells cleanly without shredding protein function.
Membrane proteins make up a third of the human proteome but show up at low concentrations and don’t dissolve easily. CHAPS helps solubilize these tricky targets for two-dimensional gel electrophoresis and Western blotting. The result: research teams identify targets for drugs or biomarkers for disease, a huge payoff for people battling conditions like cystic fibrosis or cancer.
Any scientist running mass spectrometry or capillary electrophoresis knows that cleaner samples mean more reliable data. Lipids, debris, and denatured proteins wreak havoc on delicate equipment and can ruin weeks of work. CHAPS does the dirty work of cleaning up biological prep without introducing artifacts or blocking the instruments. Peptide mapping becomes possible, and scientists can generate more accurate fingerprints for their samples.
Drug developers depend on pure, intact proteins. Years ago, I watched a whole batch of a pharmaceutical candidate crash out of solution because the wrong detergent was used. That lost time and money. CHAPS wins out by preserving the structure, allowing enzymes, receptors, and antibody fragments to keep their natural shapes. Its mildness keeps samples from foam and precipitation, making filtration and ultracentrifugation far less of a headache.
Safety matters. CHAPS tends to be less irritating than ionic surfactants. Teams don’t struggle with noxious fumes or skin burns, so workplace risk drops. Batch-to-batch consistency can’t be taken for granted in analytical chemistry. Labs demand reliable materials to compare experiments across weeks or continents; CHAPS answers that need.
Environmental impact deserves attention. Most modern manufacturers invest in greener processes and limit waste. Teams should push for transparency from suppliers to make choices that protect downstream ecosystems.
Some researchers are switching to newer detergents to address specific goals. Still, CHAPS holds an edge for isolating membrane proteins and in isoelectric focusing. For best results, teams check compatibility with their systems, test concentrations, and update protocols as new findings emerge.
1-Propanesulfonic acid, 3-(cyclohexylamino)-2-hydroxy-, monosodium salt may not get headline billing, but its impact fuels discovery in academic and industrial settings. With thoughtful use and consistent quality, it gives labs a hand in unlocking the secrets of life — and that’s something to appreciate.
Most of us remember staring at a boxy chart on the wall in our high school science classroom. Each box held a symbol—H for hydrogen, O for oxygen, C for carbon—and together, these symbols make up the language of chemistry. A compound’s chemical formula tells us who’s in the party and in what quantity. Glucose, for instance, comes out as C6H12O6. That string isn’t random; it spells out exactly how many atoms of each element decide to team up in a single molecule. The molecular weight tells us what that mix weighs in daltons or atomic mass units—roughly the grams per mole for those of us who’ve tried a lab or two.
Farmers, pharmacists, teachers, environmental watchdogs—they all end up relying on these numbers. Pick any industrial process, whether making soap or baking bread, and someone starts by weighing out precise amounts of each ingredient. Get the formula or molecular weight wrong, and you end up with something useless, maybe even dangerous. Hospitals rely on accuracy down to fractions of a gram when preparing medicines. A chemical engineer calculating how much reactant to dump into a reactor tank uses these numbers by instinct. Even bakers trust the chemical formula when mixing baking soda (NaHCO3).
Not all formulas are as simple as NaCl for table salt. Take aspirin, known to most as C9H8O4; each tablet owes its headache-busting power to this exact recipe. The molecular weight clocks in at 180.16 g/mol, which lets manufacturers stamp out millions of identical pills and pharmacists avoid dosing mistakes. Without the chemical formula and molecular weight printed right on the bottle, pharmacies couldn’t even confirm what’s in their supply.
Students hear “verify your work” from teachers, but in the lab, that habit never retires. Quality control officers in factories pull random samples and run spectral analysis to make sure actual products match theoretical formulas. Environmental analysts demand formulas and weights before testing a soil or water sample for contaminants. Regulators build safety charts—fire codes, storage rules, transport documentation—around chemical identities and their physical properties, like vapor pressure or toxicity.
So much trust in modern life circles back to these numbers. If you’ve ever worried about the air or food you consume, or the medication in your cabinet, rest easy knowing specialists with E-E-A-T credentials (expertise, experience, authority, trustworthiness) keep a close eye on these building blocks. The only way to compare apples to apples in science—evaluating dose, impact, or side effect—is to start from the same formula and weight.
Errors crop up when formula or molecular weight gets handwritten, transcribed, or entered into software. These slip-ups can lead to dangerous consequences. Strict training and regular audits offer some security. Laboratories post up-to-date charts, run controls, and use traceable sources. When companies invest in robust digital record-keeping and regular employee refreshers on chemical notation, the error rate drops. Barcode systems and automated calculators help, but human eyes remain the last line of defense.
Experience shows that making these basics visible and understandable to everyone—students, workers, policymakers—builds a safer, smarter world. The next time a chemical bottle crosses your desk or kitchen, check the label. Those numbers mean someone took the time to get things right, so life runs a little smoother, and risks shrink.
Checking if a product brings hazards isn’t just good practice—it can save lives, protect businesses, and keep the environment healthy. From household cleaners to industrial materials, certain items carry risks that often get ignored until something goes wrong. Growing up on a family farm, I saw people handle pesticides without gloves because “it’s what we always did.” Some folks ended up with irritated skin or worse. That hard lesson stuck.
Marketing rarely highlights dangers. Take drain cleaners, for example. Most use caustic chemicals that burn skin and eyes. People buy them to fix a clog and forget what could happen if the liquid splashes. American Association of Poison Control Centers reports thousands of calls each year for chemical exposure at home—children and adults alike.
Electronics also bring hidden hazards. Lithium-ion batteries, now crammed into everything from phones to bikes, can catch fire if punctured or overheated. The risk isn’t just in use—shipping and storage matter too. That’s why you see strict rules about flying with spare batteries.
Many products travel with warnings—flammable labels, corrosive pictograms, and more. Laws in the United States require companies to share safety data in the form of Safety Data Sheets (SDS). If you’ve never read one, you might be surprised by details inside. These documents list what could happen during routine use, accidents, or even if a product leaks into soil or water.
I once worked in a warehouse moving containers of isocyanates, chemicals used in foam manufacturing. We got safety training that covered everything from spills to disposal. They told us horror stories: one guy cleaned up a small spill without gear and ended up hospitalized. The message was clear—don’t guess, check the manual, and trust the gear.
Personal experience may not tell the whole story, especially with complicated substances. Even something as simple as bleach turns dangerous mixed with ammonia—an easy mistake that creates toxic gas. Hospitals see cases every year where home remedies or quick “hacks” land people in trouble.
Training and habit make a bigger difference than labels alone. OSHA studies show permanent injuries dropped in workplaces with ongoing safety education and access to equipment, not just printed warnings.
For businesses, following best practices protects employees, inventory, and reputation. It’s not just about meeting regulations; it’s about keeping people safe and avoiding lawsuits or shutdowns. Rolling out regular safety talks, keeping safety gear well-stocked, and encouraging questions pays off. No one should get punished for double-checking a procedure.
At home, simple steps go a long way. Lock up chemicals. Teach everyone—kids included—how to read labels. As someone who learned the hard way, I know most accidents come from missing information or rushed decisions. Stay curious, ask questions, and slow down before using anything new.
Hazards hide in plain sight, but knowledge, preparation, and a willingness to ask questions can keep trouble at bay. No product is completely safe or dangerous without context—how people use and store it changes everything. In my experience, diligence and understanding create a safer workspace, home, and environment for everyone.
Experience in a chemistry lab quickly teaches you that rules for chemical storage aren’t just bureaucracy; they’re about keeping yourself and your colleagues safe. 1-Propanesulfonic acid, 3-(cyclohexylamino)-2-hydroxy-, monosodium salt might sound intimidating, but treating this compound with respect makes a huge difference. While not as volatile as some reagents, this compound brings its own hazards—including possible skin and eye irritation, or health effects from inhalation.
Most raw chemicals sit on the shelves of lab storage areas until needed. For any chemical like this one, dry, well-ventilated spaces away from direct sunlight always work best. Humidity in storage areas encourages clumping and, sometimes, unwanted chemical change. Strong smells or irritating powders tend to escape when stored poorly, so a tightly sealed container pays off.
Not every lab is blessed with all the bells and whistles, but minimizing exposure to heat and moisture stands as the surest path to safe storage. Air conditioning helps keep humidity down, making it easier to store sensitive compounds. Even in a cramped corner, avoiding the impossible combination of high heat, unwanted moisture, and careless stacking of different chemicals cuts risk.
Stowing this salt next to acids or strong oxidizers leads to trouble. Once, I saw a careless mix-up between two similar-looking bottles that ended in a small but worrying release of fumes. Segregating by hazard class sounds tedious until it saves you from a real headache, literally and figuratively.
Simple solutions work as the backbone of chemical hygiene. Lab mates who label everything clearly—date, compound name, any special warnings—make the lab safer by default. Storing this compound in its own labeled, air-tight bottle stops cross-contamination and stops anyone from reaching for the wrong substance in a rush.
Nobody willingly takes a chemical splash to the face. Gloves, goggles, and coats remove most risk if anything goes wrong while handling or storing the salt. Whenever I use potent compounds, I double-check the surrounding area for spills and potential mixing.
It’s easy to focus on putting a chemical away on a shelf, but spills or waste come up sooner or later. Having a spill kit nearby—absorbent pads, pH neutralizers, gloves—turns a scary moment into an easily managed task. Following disposal procedures makes a difference down the line, especially when these compounds move into water supplies and ecosystems when handled carelessly.
Labs tracking compliance with OSHA and EPA guidelines aren’t just ticking boxes. Regulations exist because accidents aren’t rare—learning from those who made mistakes before leads to better storage practices. Checking that chemical storage is up to code avoids fines and secures the reputation of individuals and businesses alike.
Better storage starts with deliberate action. Whenever I see an organized, labeled, and clean storage area, I know its users care about safety, science, and the wellbeing of everyone in the lab. Storing 1-Propanesulfonic acid, 3-(cyclohexylamino)-2-hydroxy-, monosodium salt the right way makes all the difference—one clear label and dry spot at a time.
Some folks see the warning labels, the fine print, the “store below 25°C” instructions, and chalk it up to overcomplication. Once you've seen a product separate, solidify, or react when mixed with something it shouldn't, the importance hits home fast. No one enjoys dealing with spoiled inventory or an emergency cleanup after an unexpected reaction. For producers and users alike, keeping an eye on incompatibilities saves headaches and protects people.
A friend once mixed household bleach with a popular cleaning agent thinking a clean surface was guaranteed. Instead, the combo released dangerous chlorine gas. The lesson stuck. Incompatibility often goes beyond inconvenience—it can mean facing health hazards or total product failure. Chemicals, pharmaceuticals, food items, even consumer tech—these all show sensitivity to outside factors and mixing partners.
In my past work with adhesives and coatings, cross-checking product sheets served as the first line of defense. Heat, humidity, and trace contaminants can play havoc with product performance. Ignoring simple cautions unwittingly leads to scrapped batches or—worse—dangerous incidents. Companies learned tough lessons and spent years tightening their protocols as a result.
Some of the most troublesome issues emerge from poorly understood storage or handling. For example, pharmaceuticals may lose potency in a humid warehouse. Cleaning supplies can spoil if the cap stays loose. Food products harbor bacteria if packaged with certain plastics. If a product forms part of a system, like a resin and hardener, mixing the wrong brands leads to a sticky mess.
A look at the medical world reveals how certain antibiotics and vitamins break down faster when exposed to light or air. Electronics manufacturers meticulously test plastics and metals for sensitivity to temperature shifts since a warped connector leaves a device useless.
The American Chemical Society has documented incidents where one overlooked incompatibility led to blown pipes, laboratory fires, and personal injuries. The FDA and EPA issue regular updates on product recalls where improper formulation or storage created risks. Product instability can happen in subtle ways too, with change in color, smell, or texture serving as early warnings. Every label, safety data sheet, and technical spec sheet tells a story written by someone who had to learn things the hard way.
Scientists look carefully at pH levels, moisture content, even the type of packaging material. A bottle made from polyethylene permits slow oxygen transfer, slowly degrading what’s inside. Tiny details matter—combining vitamin C tablets with iron supplements, for instance, creates oxidation that saps both nutrients.
Everyone wins by adopting a culture of careful product stewardship. Employees can commit to logging storage temperatures and inspecting packaging. Producers should share specifics on what not to mix, or how sunlight ruins shelf life. Even simple tools, like direct-from-the-supplier compatibility checkers or QR codes on labels, can reduce guessing. Forgoing shortcuts—refusing to eyeball compatibility or ignore obvious warning signs—makes modern workplaces safer for staff and customers alike. Trust the experts, read the materials, and never underestimate what can go wrong if the basics get ignored.
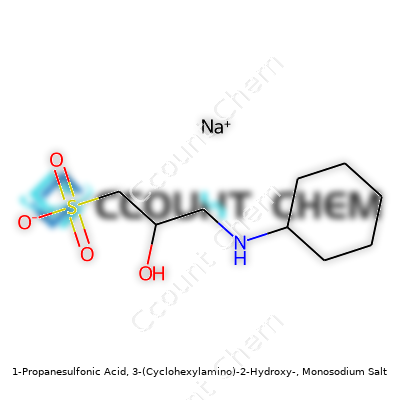

| Names | |
| Preferred IUPAC name | sodium 3-(cyclohexylamino)-2-hydroxypropane-1-sulfonate |
| Other names |
CHAPS 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate 3-[(3-Cholamidopropyl)dimethylammonio]propanesulfonic acid CHAPS sodium salt 3-(Cyclohexylamino)-2-hydroxy-1-propanesulfonic acid sodium salt CAS 11374-19-7 |
| Pronunciation | /prə-ˈpeɪn-sʌl-ˈfoʊ-nɪk ˈæs.ɪd θri ˌsaɪ-kləˈhɛk-səl-əˈmiː-noʊ ˈtuː ˈhaɪ-drɒk-si ˌmɒn-oʊˈsoʊ-di-əm sælt/ |
| Identifiers | |
| CAS Number | 42174-52-9 |
| 3D model (JSmol) | `/cyqvL8TxDRGBwPYJUHnGkIRlJzTdlA6IXdNnoy2cuglRez2oJ6k2RFxYDs2fzGEGZm4G6dkprdFMn/hTyBC0bQ==` |
| Beilstein Reference | 104388 |
| ChEBI | CHEBI:91298 |
| ChEMBL | CHEMBL3185063 |
| ChemSpider | 20742112 |
| DrugBank | DBSALT001122 |
| ECHA InfoCard | 03e76244-6db0-4d4d-849e-3cccac08c3fe |
| EC Number | 246-807-3 |
| Gmelin Reference | 96974 |
| KEGG | C16452 |
| MeSH | D041089 |
| PubChem CID | 124875 |
| RTECS number | YG8570000 |
| UNII | PXI5S5579X |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7031653 |
| Properties | |
| Chemical formula | C9H18NO4SNa |
| Molar mass | 277.35 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.23 g/cm3 |
| Solubility in water | soluble |
| log P | -2.7 |
| Vapor pressure | 0.0000135 mmHg at 25°C |
| Acidity (pKa) | pKa = 1.53 |
| Basicity (pKb) | pKb = 5.43 |
| Magnetic susceptibility (χ) | -67.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.523 |
| Dipole moment | 7.9161 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 268.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -902.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | > 213.7 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2550 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not Established |
| IDLH (Immediate danger) | NO DATA |
| Related compounds | |
| Related compounds |
Choline 3-cyclohexylamino-2-hydroxypropanesulfonate Propanolol sulfate Propanolol hydrochloride 3-(Cyclohexylamino)-2-hydroxy-1-propanesulfonic acid 3-(Cyclohexylamino)-2-hydroxypropanesulfonic acid sodium salt |