Looking at the story of 1-octanesulfonic acid, you trace the shifting landscape of analytical and synthetic chemistry over the last several decades. In the late twentieth century, researchers needed ways to fine-tune separation methods like high-performance liquid chromatography (HPLC). Somewhere in the 1970s, people discovered that compounds like 1-octanesulfonic acid acted as “ion-pairing” agents, smoothing out tricky analyses of basic drugs and amines. By the 1980s, catalogs listed this sulfonate as an off-the-shelf solution for chemists trying to boost their results. The industry has found steady demand ever since, especially in pharmaceutical analysis circles.
1-Octanesulfonic acid, better known in some labs as sodium octane sulfonate, comes as a white crystalline powder. It stands out for its role in reversing-phase chromatography, helping folks tweak selectivity and retention times. Thanks to its straightforward formula, it carves out space in applications that can’t tolerate more reactive or aromatic sulfonates. The sodium salt usually appears in chemical supply catalogs, alongside food-grade and high-purity forms for varied requirements across research and quality control.
The acid form has a melting point around 70°C to 76°C, showing moderate stability under routine storage. It dissolves well in water, producing clear and slightly acidic solutions. The molecule carries a straight-chain alkyl group, which produces a subtle shift from shorter sulfonic acids; its hydrocarbon tail changes how it interacts with organic analytes and separation phases. You also notice a little bit of odor, recognizable to anyone who’s spent hours hunched over a bench pouring out small lots. Chemically non-volatile, it handles gentle heating, but care matters since significant decomposition kicks in at high temperatures or under harsh acidic or basic conditions.
Chemists working with 1-octanesulfonic acid expect a product that reaches at least 98% purity on a dry basis. Impurities and moisture content count, especially in pharmaceutical contexts, since inconsistency impacts method validation. Safety data sheets underline risks if you swallow or inhale it, but the relatively low acute toxicity means it fits most standard risk codes. Packaging tends to favor sealed glass or polyethylene containers, with clear labeling on net weight, batch, CAS number (5324-84-5), and hazard marks. That information streamlines audits and supports compliance with ISO and GMP frameworks.
Most commercial production works by sulfonating 1-octanol using sulfonic acid or sulfur trioxide—classic chemistry for transforming alcohols into sulfonic acids. Pilot plants control temperature and pressure, ensuring high yields and lower byproduct formation. Operators often neutralize the acid with sodium hydroxide to make the sodium salt, which improves shelf stability and makes the product easier to weigh, dissolve, and ship. Quality assurance teams handle rigorous titration, purity checks by NMR and HPLC, and sometimes limit tests for metals or non-volatile residue based on the customer’s needs.
This sulfonic acid group brings solid water solubility and a strong acidic character. The molecule withstands a range of pH changes in solution while keeping the octane backbone intact. Chemists use 1-octanesulfonic acid or its sodium variant to pair with cationic analytes for HPLC, relying on the long hydrophobic tail to drive interactions without introducing aromatic groups that complicate UV detection. Creative modification remains an option; derivatization of the octane group or sulfonate esterification emerges in specialty synthesis, sometimes leading to tailored surfactants or ionic liquids with custom wetting or separation abilities.
You run across several names depending on source and geography. Sodium-1-octanesulfonate, n-octanesulfonic acid, and simply “OSA” get tossed around in lab meetings and purchase orders. For those importing and exporting, regulatory agencies reference all common synonyms, and labeling reflects these conventions clearly to avoid mix-ups. Some suppliers market the pure acid under the “sulfonic acid, C8” title, or bundle it with analytical columns as a specialty additive kit, but underlying molecule stays consistent.
Working with 1-octanesulfonic acid doesn’t call for heavy-duty controls you’d need for toxic or highly reactive chemicals, but it’s no cakewalk either. Protective eyewear, gloves, and lab coats prevent skin and eye contact, and most companies set up local ventilation above scales and wet benches. Ingestion risks show up at gram quantities or higher; the main health risk involves local irritation rather than systemic toxicity. Emergency procedures boil down to rinsing affected areas, avoiding inhalation of fine dust, and storing away from strong bases or oxidizing agents. Waste streams get neutralized, diluted, and routed through standard lab disposal services, hitting regional environmental compliance rules for sulfonates.
1-Octanesulfonic acid plays a quiet but essential part in pharmaceutical testing. Analysts rely on its ion-pairing function to separate basic amines and peptides during method development. It steps up in quality control of active ingredients, stability monitoring, and even some food safety labs for amino acid and preservative analysis. Water testing, biotechnology, and specialty surfactant research also use its properties, benefitting from the blend of aqueous solubility and hydrocarbon length. Some seasoned chemists remember using it for specialty cleaning or calibration tweaks, and today’s suppliers cater to both academic and industrial buyers tuning machines for daily problem-solving.
Lab teams in pharmaceutical, biotech, and chemical fields keep digging into adjustments on the alkyl sulfonic acid template. The drive comes from ever-changing regulations and the push for higher sensitivity, lower detection limits, or reduced background in mass spectrometry. Recent academic work tries out chain-length modifications and blends with other mobile phase additives, looking to sharpen signals or reduce run times. Environmental chemists test the fate of alkyl sulfonates during advanced oxidation and bioremediation, weighing their effect on aquatic life and drinking water.
Toxicology databases tag 1-octanesulfonic acid with a low acute toxicity score, though everything changes with concentration and chronic exposure. Studies on aquatic organisms reveal moderate effects at high levels, leading regulators to keep tabs on its discharge in wastewater. Research from the 2000s and beyond flags some concern over persistent bioaccumulation potential for sulfonated surfactants in general, highlighting the need for detailed fate and transport studies. Human health reviews stay cautious, because, like most chemicals with strong acids in the formula, the threat mostly lies in corrosive injury to tissue rather than systematic poisoning. That said, safety margins hold up in typical laboratory and industrial handling.
Analytical and synthetic chemists show no signs of moving past 1-octanesulfonic acid anytime soon. As HPLC and MS instrumentation keeps evolving, the search for subtler, more selective additives builds on the foundation laid by this molecule. Environmental researchers stay busy mapping its long-term impact, driving interest in greener modifications and degradable analogs that won’t build up in soil or groundwater. Advances in supramolecular chemistry even suggest new uses, such as templating for functional materials or tuning self-assembly in nanoscience. Long story short, anyone with a hand in wet chemistry or analytical method development stands to cross paths with this straightforward yet adaptable sulfonic acid, just as those before them have for half a century.
Chemistry sometimes throws around names that never make it onto household labels. 1-Octanesulfonic acid is one of those chemicals that rarely gets talked about outside of labs, but it holds a quiet importance in a number of places, including food safety, pharmaceuticals, and chemical analysis. My own experience working in a university laboratory showed me how certain compounds don’t make headlines, yet without them, many of the things we trust every day would lose a layer of safety and precision.
I first encountered 1-octanesulfonic acid while helping with water quality assessments. We ran a technique called high-performance liquid chromatography (HPLC). HPLC separates out molecules in complex mixtures so scientists can check for things like contaminants or the exact makeup of a medicine. 1-Octanesulfonic acid turns out to be a valuable “ion-pairing agent.” Basically, it teams up with charged particles, letting those particles move differently through the system, so instruments spot them more easily. This matters most when dealing with tricky molecules that don’t want to stay apart or behave well in analysis.
Every sandwich you eat, every glass of juice you pour—food and drink get checked for tiny amounts of pesticides, preservatives, or even unintentional additives. The straightforward names on nutrition labels always made me think food safety happened in the kitchen, but sitting behind a computer running data, I learned it’s chemicals like 1-octanesulfonic acid that help labs target unsafe ingredients. In these tests, analysts spike a sample with 1-octanesulfonic acid, fine-tune the HPLC, then pick out harmful residues or unauthorized additives that could go unnoticed without such tools.
Drug developers never gamble on guesses. If you’re picking up a prescription or buying over-the-counter medication, the purity matters every time. 1-Octanesulfonic acid helps break down complicated drug ingredients during quality control. Take antibiotics, for example. Some antibiotics break apart into similar-looking components that hide from detection. This acid works as a partner to keep those parts separate during testing, so the final pill holds only what’s on the label—nothing more, nothing less.
Every chemical tool comes with its own set of risks. Sulfonic acids aren’t substances for casual use. They need good ventilation and protective equipment during handling, and lab techs keep training up-to-date for safety. Proper disposal is a big deal to keep the environment protected. I saw firsthand that a lab’s job isn’t done until every drop is accounted for. Regulations keep everyone honest and the environment cleaner.
As laboratories work with more complex samples, demand for sensitive and reliable analysis grows. Innovation around safer ion-pairing agents continues, but 1-octanesulfonic acid still fills key roles that haven’t found strong substitutes. Investment in staff safety training, recycling systems for chemicals, and development of less hazardous analytical agents can shape a future where science keeps improving people’s health and daily life, while shrinking risks along the way.
1-Octanesulfonic acid finds its place in lab work, especially among folks running high performance liquid chromatography (HPLC). Its role as an ion-pairing agent makes it valuable for separating and analyzing certain compounds. A lot of researchers and technicians rely on its straightforward chemistry and the consistency that it offers.
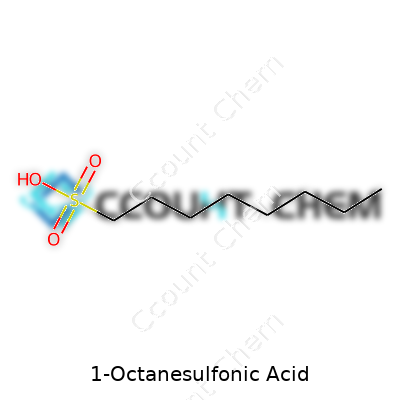
The formula, C8H18O3S, lays out the structure of 1-Octanesulfonic acid: eight carbons, eighteen hydrogens, three oxygens, and one sulfur atom. The “octane” tells us there are eight carbons in a straight chain. Chemists often focus on the sulfonic acid group (-SO3H) at the end of this chain. That group brings strong acid properties and increases solubility in water, key for its use in science labs.
Many labs use 1-octanesulfonic acid because some molecules refuse to separate cleanly on their own. Tough-to-handle molecules such as certain pharmaceuticals and peptides end up splitting apart with less trouble when this acid is present. Those working in analytical chemistry know how much time and frustration a good separation technique can save.
I’ve noticed that students and newcomers can get tripped up on naming and formulas. Memorizing “octane” can remind you of gasoline, but toss in “sulfonic acid” and it feels miles away from the gas station. In truth, it's the chemical group that flips it from a basic oil component into something useful for scientists and manufacturers. Slipping up on the formula can throw off important experiments, which is why careful double-checking pays off.
Most work with 1-octanesulfonic acid involves tiny amounts, but strong acids always deserve respect. Gloves and goggles aren’t optional, because contact can irritate skin and eyes. Good ventilation in a lab space is standard practice for a reason. Years in the lab have taught me not to make assumptions about chemical safety, no matter how familiar a substance seems.
Industries needing reliable chromatography look to compounds like 1-octanesulfonic acid to deliver steady results. Pharmaceutical companies, for instance, count on precise analysis during drug development. Mistakes with an ion-pairing reagent can delay timelines and add extra cost in already complicated processes.
The chemical formula plays another subtle, but crucial, role in sourcing and purchasing. No engineer or lab manager wants to order a batch with the wrong number of carbons, and a single slip in the molecular structure changes everything. This goes for researchers checking the purity of reagents, too. Purity checks help verify that a bottle contains only what the label claims, preventing failed experiments and wasted hours.
Greater access to clear reference materials can prevent mistakes related to chemical formulas. Chemistry textbooks, online databases, and supply catalogues all offer confirmation for anyone uncertain about structural details or practical uses. In the past, colleagues in my own lab swapped notes and kept a ready list of commonly-used formulas taped to storage cabinets, which acted as a constant safety net. Sharing these habits with new lab members is one way to keep accidents and errors at bay.
Training that stresses both safety and chemical literacy gives everyone more confidence. Knowing the story behind the formula helps people remember it, use it wisely, and understand why any small error in those letters and numbers could cause big headaches down the line.
1-Octanesulfonic acid belongs to a family of organic sulfonic acids commonly used in the lab. Most folks in research or chemical analysis may have come across the compound as an ion-pairing agent, especially in HPLC (High Performance Liquid Chromatography) methods.
Mistakes around chemical safety tend to pop up most when labels get ignored. I remember working in the lab as a research assistant, watching bottles pass hands faster than safety data sheets ever got read. The same can happen with 1-octanesulfonic acid. Though not as notorious as hydrofluoric acid or stronger mineral acids, this chemical isn’t an everyday kitchen item. The acid can irritate skin or eyes, and if someone breathes in dust or fumes, respiratory discomfort follows. Swallowing the stuff by accident leads to a rough time, so storing it far away from food or drinks makes sense.
The effects come down to the sulfonic acid group. These molecules don’t easily catch fire and don’t explode, but they do break down into other stuff when heated—sometimes letting off gases you don’t want in your nose or lungs. Handling dry powder or concentrated solutions means gloves and goggles should stay on. The Material Safety Data Sheet (MSDS) available from trusted chemical suppliers like Sigma-Aldrich or Thermo Fisher spells these focus points out. Simple steps like keeping a well-ventilated workspace or using a fume hood protect everyone nearby.
Hazard ratings usually land 1-octanesulfonic acid in a moderate risk category. The chemical does not reach the hazardous heights of corrosive strong acids like hydrochloric or sulfuric. Still, irritation pops up more than most would expect—not deadly, just bothersome, and that counts for routine lab safety. One animal study I read found moderate irritation on rabbit skin, but not permanent harm. The compound doesn’t show up on major international lists as carcinogenic or highly toxic.
Long-term exposure rarely happens outside chemical plants or research facilities. Chronic health effects haven’t made headlines so far. Folks with sensitive skin or a habit of skipping gloves would likely feel discomfort, though. And just because something escapes regulatory lists doesn’t guarantee zero risk, especially with repeat exposure.
Chemicals like 1-octanesulfonic acid don’t just vanish after hitting a drain. Water treatment plants aren’t magic soakers for organic acids. I remember the mess caused when a neighboring lab dumped their run-off without neutralizing acids. The stench was a good reminder that small actions multiply fast. Although 1-octanesulfonic acid isn’t a major high-tox animal killer, it does break down slowly and can hang around in water or soil, slowly affecting plants or tiny organisms. Proper disposal means neutralizing with a base and collecting waste in regulated chemical containers.
Anyone using 1-octanesulfonic acid must trust more than a printed label. Proper chemical storage, smart workspace layout, and a ready eye-wash station close at hand matter more than speed. Education pushes safety further than labels. Using clear Standard Operating Procedures (SOPs) and regular safety drills help avoid near-misses. If something spills or splashes, quick cleanup with the right neutralizing agent and a willingness to swap out gloves or goggles makes the difference.
Companies and labs could do better by tracking amounts used, limiting exposures, and maybe considering alternative additives if safer options exist. In research, the sharpest minds aren’t the ones who take shortcuts—they’re the ones who respect the sharp corners hidden in even “moderately hazardous” chemicals like 1-octanesulfonic acid.
Anyone fitting out a lab or running a chemical warehouse learns fast that certain acids challenge your routine. 1-Octanesulfonic acid isn’t the flashiest reagent, but it has a reputation for turning sloppy storage into real headaches. I’ve witnessed damage to shelving, accidental leaks, and lost batches—all because the basics of acid storage got ignored or overly complicated. Keeping things simple and practical helps maintain safety without fuss.
Steel shelves look durable, yet repeated contact with acids like this one shortens their lifespan. I’ve seen pitted, rusty trays after months of poor storage. Polyethylene and glass have always handled the job better. Containers need intact seals. No one wants a sulfur smell creeping into the room just because a cap didn’t fit snugly or a weak plastic bottle started weeping.
A dry, cool spot, away from direct sunlight, keeps the acid stable. Heat speeds up decomposition and can make fumes more noticeable. Hot storerooms interact poorly with acids, contributing to warped plastic drums and dried-out seals—a problem that’s easy to overlook during warm spells.
Mixing acids and bases seems innocent until pressure builds up in a closed bottle and a shelf is ruined. Peroxides, oxidizers, and strong bases shouldn’t come near your stash. People learn this after minor accidents ruin whole batches of chemicals, leading to lost money and wasted time. Labels help, but real safety comes from separating shelves and using color-coded bins.
Workers tend to skip opening vents or using extraction fans on slow days. Then, a strong smell of sulfur hits during an unexpected spill. Good airflow matters because even small leaks or open bottles can create an unpleasant environment. Emergency eyewash stations and clean-up kits with absorbent neutralizers belong close by. Training helps everyone spot issues, so nobody fumbles around if something leaks or splashes.
Many countries keep chemical regulations strict for good reason. I remember an inspection in my early days that pulled up carelessly stacked acid bottles and taught us a real lesson—store acids below eye level, use clear labels, and keep quantities low wherever possible. Every inspection since, we’ve stuck to these basics and saved ourselves from trouble and fines.
Manufacturers may offer added safety insight in their technical documents—a quick read can stop mistakes before they start. I never skip checking these whenever a new drum comes in. Colleagues at other labs have sometimes missed a warning and gotten a nasty surprise as a result.
A few simple habits—using the right shelves, clear separation, solid container checks, and basic ventilation—go a long way with 1-octanesulfonic acid. Attention to these things keeps everyone safer, spares equipment, and stops small errors from becoming bigger disasters. The goal isn’t just meeting rules, but protecting your investment and the people who share the space.
1-Octanesulfonic acid plays a big role in labs using high performance liquid chromatography (HPLC), especially for those needing a solid ion-pairing agent. Purity levels guide how well it does its job. If you’ve ever run an HPLC test, you might have seen how trace impurities can skew a whole project, leave ghosts in the background, or mess with your baseline. Lab-grade and analytical work really show just how picky chemists need to be about what goes into their samples.
There is no universal standard for every single application—what really matters is matching the grade to your task. For example, routine separations might get by with a lower grade, but drug quality control calls for much higher purity.
Manufacturers usually offer 1-octanesulfonic acid in a couple of main purity categories. Most labs see material at about 98% up to 99%. For a lot of method development in chromatography, this works well. You find this grade in reagent bottles marked “for synthesis” or “technical,” and it handles routine ion-pairing with few surprises. Factories can make and ship this pretty efficiently, so it’s what many academic and industrial users end up selecting.
Analytical grade, though, shoots higher. Here, the percentage climbs to 99.5% or better. In practice, these batches include less water, fewer unknown bits, and minimal sodium or iron. Analytical runs in pharma, food testing, or toxicology chase small signals, so they lean on this high-purity material. Some suppliers share certificates of analysis pinpointing exact percentages, which gives extra confidence.
Impurities pop up from the manufacturing process itself or from storage. Sulfonic acids often pull in water from the air, and even trace metals may hitch a ride from contact with equipment. In HPLC, stray ions or organics creep into the baseline or gum up sensitive detectors. If you’ve watched a chromatogram shift or flip between runs, you know the headache of not knowing where the problem started. High-purity material weeds out some of that risk.
Labs must also look at “suitability for use.” It’s easy to think purity numbers say everything, but compatibility with other solvents, ease of dissolving, and quick handling still count for a lot. I’ve worked with older material that technically met the percentage but needed more cleanup after being exposed to the air or the wrong container.
Most daily work in research, teaching, or industry runs on the 98%-99% grade without a hitch. Specialty tasks—such as regulatory submissions or work with trace contaminants—benefit from the purer, pricier grade. I’ve found that asking for the supplier’s certificate helps spot hidden metals or moisture problems before they throw off sensitive tests.
Labs can stretch budgets by storing high-purity portions sealed and only opening what’s needed, keeping moisture at bay. It makes sense to check the source, too. Reputable suppliers publish their purity standards online, and quality control teams regularly confirm batch data.
If you’ve ever lost hours to a contaminated standard or scrambled to explain noisy data, you already know the value of choosing the right grade and handling it with respect.
| Names | |
| Preferred IUPAC name | octane-1-sulfonic acid |
| Other names |
Octane-1-sulfonic acid n-Octanesulfonic acid 1-Octanesulphonic acid n-Octylsulfonic acid |
| Pronunciation | /wʌn-ɒkˈteɪnˌsʌlˌfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 126-68-1 |
| Beilstein Reference | 1713881 |
| ChEBI | CHEBI:37918 |
| ChEMBL | CHEMBL20438 |
| ChemSpider | 16594 |
| DrugBank | DB04519 |
| ECHA InfoCard | 03d181b6-3f81-469a-8faf-c0b7472f466d |
| EC Number | EC 219-077-6 |
| Gmelin Reference | 72370 |
| KEGG | C01733 |
| MeSH | D017760 |
| PubChem CID | 8773 |
| RTECS number | RG1630000 |
| UNII | 8KQ32A50Z2 |
| UN number | UN2586 |
| Properties | |
| Chemical formula | C8H18O3S |
| Molar mass | 208.32 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.14 g/cm3 |
| Solubility in water | soluble |
| log P | -0.75 |
| Vapor pressure | 1.55E-7 mmHg at 25°C |
| Acidity (pKa) | 1.80 |
| Basicity (pKb) | 1.54 |
| Magnetic susceptibility (χ) | -5.1e-6 |
| Refractive index (nD) | 1.439 |
| Viscosity | 22 cP (20°C) |
| Dipole moment | 2.39 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 251.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1136.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5635.1 kJ/mol |
| Pharmacology | |
| ATC code | V03AE03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P280: Wear protective gloves/protective clothing/eye protection/face protection. P261: Avoid breathing dust/fume/gas/mist/vapours/spray. P264: Wash hands thoroughly after handling. P301+P312: IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 110 °C |
| Autoignition temperature | 300 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1450 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1450 mg/kg |
| NIOSH | WYQ4377F7A |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 g/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Benzenesulfonic acid Alkylsulfonic acids |